Abstract
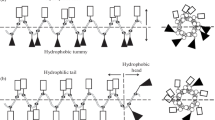
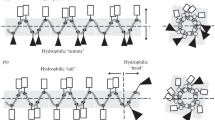
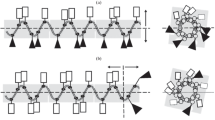
Comparative antimicrobial properties of three artificial cationic synthetic antimicrobial peptides (SAMP): (RAhaR)4AhaβA (where R is Arg, Aha is 6-aminohexanoic acid, βA is beta-alanine), (KFF)3K and R9F2 with various amphiphilic properties have been studied relative to pathogenic strains of microorganisms: Gram-negative bacteria Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, and Salmonella enterica, Gram-positive bacteria Staphylococcus aureus, and pathogenic yeast fungus Candida albicans. The selectivity index (SI) values of the peptide preparations were calculated as the ratio of the 50% cytotoxic concentration (TC50) towards eukaryotic host cells to the MIC50 values of the testing antimicrobial peptides. The studied SAMPs appeared to be the most active against the pathogenic yeast fungus C. albicans and the bacterial strains St. aureus and P. aeruginosa. The SI values in these cases exceed 40. Some assumed molecular interactions of the studied SAMPs on the microbial cells have been considered, and possible pathways to increase their antimicrobial activity have been suggested. The proposed SAMPs can serve as a basis for the design and synthesis of new promising synthetic antimicrobial agents.
Similar content being viewed by others
Abbreviations
- aa:
-
amino acid
- AMP:
-
antimicrobial peptides
- MIC50 :
-
minimum inhibitory concentration, which inhibits the growth of microorganisms by 50%
- MTT:
-
3-(4,5-dimethylthiazsol-2-yl)-2,5-diphenyltetrasolium bromide
- SAMP:
-
synthetic antimicrobial peptides
- Aha:
-
6-aminohexanoic acid
- βА:
-
betaalanine
- ESI-MS:
-
electrospray ionization mass spectrometry
- MALDI-TOF MS:
-
Matrix assisted laser desorption ionizationtime of flight mass spectrometry
- SI:
-
selectivity index
- TC50 :
-
50% cytotoxic concentration
References
Bahar, A.A. and Ren, D., Pharmaceuticals (Basel), 2013, vol. 6, no. 12, pp. 1543–1575.
Peschel, A. and Sahl, H.G., Nat. Rev. Microbiol., 2006, vol. 4, no. 7, pp. 529–536.
Chung, P.Y. and Khanum, R., J. Microbiol. Immunol. Infect., 2017, vol. 50, no. 4, pp. 405–410.
Bechinger, B. and Gorr, S.U., J. Dent. Res., 2017, vol. 96, no. 3, pp. 254–260.
Mahlapuu, M., Hakansson, J., Ringstad, L., and Bjorn, C., Front. Cell. Infect. Microbiol., 2016, vol. 6, p. 194. doi 10.3389/fcimb.2016.00194
Balandin, S.V. and Ovchinnikova, T.V., Russ. J. Bioorg. Chem., 2016, vol. 42, no. 3, pp. 229–248.
Brown, K.L. and Hancock, R.E., Curr. Opin. Immunol., 2006, vol. 18, no. 1, pp. 24–30.
Azimova, V.T., Potaturkina-Nesterova, N.I., and Nesterov, A.S., Modern Probl. Sci. Education, 2015, no. 1. https://science-education.ru/ru/article/view?id=17746. Accessed November 10, 2017.
Jenssen, H., Hamill, P., and Hancock, R.E., Clin. Microbiol. Rev., 2006, vol. 19, no. 3, pp. 491–511.
Yeaman, M.R. and Yount, N.Y., Pharmacol. Rev., 2003, vol. 55, no. 1, pp. 27–55.
Balandin, S.V. and Ovchinnikova, T.V., Russ. J. Bioorg. Chem., 2016, vol. 42, no. 4, pp. 343–360.
Matsuzaki, K., Biochim. Biophys. Acta, 1999, vol. 1462, nos. 1–2, pp. 1–10.
Okorochenkov, S.A., Zheltukhina, G.A., and Nebol’sin, V.E., Biochemistry (Moscow), Suppl. Ser. B: Biomed. Chem., 2011, vol. 5, no. 2, pp. 95–102.
Bilikova, K., Huang, S.C., Lin, I.P., Simuth, J., and Peng, C.C., Peptides, 2015, vol. 68, pp. 190–196.
Panteleev, P.V., Bolosov, I.A., Balandin, S.V., and Ovchinnikova, T.V., J. Pept. Sci., 2015, vol. 21, no. 2, pp. 105–113.
Giuliani, A., Pirri, G., and Nicoletto, S.F., Cent. Eur. J. Biol., 2007, vol. 2, no. 1, pp. 1–33.
Oren, Z. and Shai, Y., Biochemistry, 1997, vol. 36, no. 7, pp. 1826–1835.
Chen, Y., Mant, C.T., Farmer, S.W., Hancock, R.E., Vasil, M.L., and Hodges, R.S., J. Biol. Chem., 2005, vol. 280, no. 13, pp. 12316–12329.
Powers, J.P. and Hancock, R.E., Peptides, 2003, vol. 24, no. 11, pp. 1681–1691.
Eisenberg, D., Weiss, R.M., and Terwilliger, T.C., Nature, 1982, vol. 299, no. 5881, pp. 371–374.
Schiffer, M. and Edmundson, A.B., Biophys. J., 1967, vol. 7, no. 2, pp. 121–135.
Oren, Z. and Shai, Y., Biopolymers, 1998, vol. 47.
Eisenberg, D., Annu. Rev. Biochem., 1984, vol. 53, pp. 595–623.
Ghosal, A. and Nielsen, P.E., Nucl. Acids Ther., 2012, vol. 22, no. 5, pp. 323–334.
Mellbye, B.L., Puckett, S.E., Tilley, L.D., Iversen, P.L., and Geller, B.L., Antimicrob. Agents Chemother., 2009, vol. 53, no. 2, pp. 525–430.
Moulton, H.M., Nelson, M.H., Hatlevig, S.A., Reddy, M.T., and Iversen, P.L., Bioconjug. Chem., 2004, vol. 15, no. 2, pp. 290–299.
Turner, J.J., Arzumanov, A.A., and Gait, M.J., Nucleic Acids Res., 2005, vol. 33, no. 1, pp. 27–42.
Shai, Y., Biochim. Biophys. Acta, 1999, vol. 1462, nos. 1–2, pp. 55–70.
Pacor, S., Giangaspero, A., Bacac, M., Sava, G., and Tossi, A., J. Antimicrob. Chemother., 2002, vol. 50, no. 3, pp. 339–348.
Silva, A., Jr. and Teschke, O., Biochim. Biophys. Acta, 2003, vol. 1643, nos. 1–3, pp. 95–103.
Jacobsen, F., Mohammadi-Tabrisi, A., Hirsch, T., Mittler, D., Mygind, P.H., Sonksen, C.P., Raventos, D., Kristensen, H.H., Gatermann, S., Lehnhardt, M., Daigeler, A., Steinau, H.U., and Steinstraesser, L., J. Antimicrob. Chemother., 2007, vol. 59, no. 3, pp. 493–498.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.V. Amirkhanov, N.V. Tikunova, D.V. Pyshnyi, 2018, published in Bioorganicheskaya Khimiya, 2018, Vol. 44, No. 5, pp. 492–505.
Rights and permissions
About this article
Cite this article
Amirkhanov, N.V., Tikunova, N.V. & Pyshnyi, D.V. Synthetic Antimicrobial Peptides: I. Antimicrobial Activity of Amphiphilic and Nonamphiphilic Cationic Peptides. Russ J Bioorg Chem 44, 492–503 (2018). https://doi.org/10.1134/S1068162018050035
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162018050035