Abstract
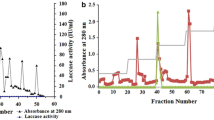
A laccase from the culture filtrate of white rot fungus Daedalea flavida MTCC-145 has been purified and characterized. The method involved concentration of the culture filtrate by ultrafiltration and an anion exchange chromatography on diethylaminoethyl (DEAE) cellulose. The sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and native polyacrylamide gel electrophoresis (native PAGE) both gave single protein bands indicating that the enzyme preparation was pure. The molecular mass of the enzyme determined from SDS-PAGE analysis was 75.0 kDa. Purification fold was 21.5 while recovery of the enzyme activity was 11.52%. Using 2,6-dimethoxyphenol, diammonium salt of 2,2'-[azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid)] and 3,5-dimethoxy-4-hydroxybenzaldehyde azine as substrates, the Km, kcat, and k cat/K m values of the laccase were found to be 440 µM, 6.45 s–1, 1.47 × 104 M–1 s–1; 366 µM, 6.45 s–1, 1.76 × 104 M–1 s–1; and 226 µM, 6.45 s–1, 2.85 × 104 M–1 s–1, respectively. The pH and temperature optima were 4.5 and 50°C, respectively. The enzyme was most stable at pH 5.0 when exposed for 1 h. The purified laccase has yellow color and shows no absorption band around 610 nm characteristic of blue laccases. The enzyme transforms toluene and substituted toluenes to corresponding benzaldehyde and substituted benzaldehydes in the absence of mediator molecules with higher catalytic efficiency as compared to other known laccases.
Similar content being viewed by others
References
Multi-Copper Oxidases, Messerschmidt, A., Ed., Singapore: World Scientific Publishing, 1997.
Yadav, M. and Yadav, K.D.S., Structural and functional aspects of lignolytic enzymes, in Lignocellulose Biotechnology: Future Prospects, Kuhad, R.C., and Singh, I.K., Eds., New Delhi: International Publishing House, Pvt. Ltd., 2007, pp. 63–88.
Riva, S., Trends Biotechnol., 2006, vol. 24, pp. 219–226.
Baldrian, P., FEMS Microbiol. Rev., 2006, vol. 30, pp. 215–242.
Dwivedi, U.N., Singh, P., Pandey, V.P., and Kumar, A., J. Mol. Cat. B: Enzymatic, 2011, vol. 68, pp. 117–128.
Quintanar, L., Yoon, J., Aznar, C.P., Palmer, A.E., Andersson, K.K., Britt, R.D., and Solomon, E.I., J. Am. Chem. Soc., 2005, vol. 127, pp. 13832–13845.
Yoshida, H., J. Chem. Soc., 1883, vol. 43, pp. 472–486.
Bao, W., Mally, D.M.O., Whetten, R., and Sederoff, R.R., Science, 1973, vol. 260, pp. 672–674.
Huang, H.W., Zoppellero, G., and Sakurai, T., J. Biol. Chem., 1999, vol. 274, pp. 3271–3272.
Chaurasia, P.K., Yadav, A., Yadav, R.S.S., and Yadava, S., Res. Rev. Biosci., 2013, vol. 7, pp. 66–71.
Chaurasia, P.K., Bharati, S.L., and Singh, S.K., Res. Plant Sci., 2013, vol. 1, pp. 32–37. doi: 10.12691/plant1-2-5
Messerschmidt, A. and Huber, R., Eur. J. Biochem., 1990, vol. 187, pp. 341–352.
Mayer, A.M. and Staples, R.C., Phytochemistry, 2002, vol. 60, pp. 551–565.
Enguita, F.J., J. Biol. Chem., 2004, vol. 279, pp. 23472–23476.
Thomas, B.R., Yonekura, M., Morgan, T.D., Czapla, T.H., Hopins, T.L., and Kramer, K.J., Biochemistry, 1989, vol. 19, pp. 611–622.
Parkinson, N., Smith, I., Weaver, R., and Edwards, J.P., Insect Biochem. Mol. Biol., 2001, vol. 31, pp. 57–63.
Morozova, O.V., Shumakovich, G.P., Gorbacheva, M.A., Shleev, S.V., and Yaropolov, A.I., Biochemistry, 2007, vol. 72, no. 10, pp. 1136–1150.
Edens, W.A., Goins, T.Q., Dooley, D., and Henson, J.M., Appl. Environ. Microbiol., 1999, vol. 65, no. 7, pp. 3071–3074.
Iyer, G. and Chattoo, B.B., FEMS Microbiol. Lett., 2003, vol. 227, no. 1, pp. 121–126.
Binz, T. and Canevascini, G., Curr. Microbiol., 1997, vol. 35, no. 5, pp. 278–281.
Palonen, H., Saloheimo, M., Viikari, L., and Kruus, K., Enz. Microb. Technol., 2003, vol. 33, no. 6, 854–862.
Kiiskinen, L.L., Viikari, L., and Kruus, K., Appl. Microbiol. Biotechnol., 2002, vol. 59, nos. 2–3, pp. 198–204.
Thakker, G.D., Evans, C.S., and Rao, K.K., Appl. Microbiol. Biotechnol., 1992, vol. 37, no. 3, pp. 321–323.
Froehnerand, S.C. and Eriksson, K.E., J. Bacteriol., 1979, vol. 120, no. 1, pp. 458–465.
Molitoris, H.P. and Esser, K., Archiv. für Mikrobiologie, 1970, vol. 72, no. 3, pp. 267–296.
Banerjee, U.C. and Vohra, R.M., Folia Microbiologica, 1991, vol. 36, no. 4, pp. 343–346.
Rodriguez, A., Falcon, M.A., Carnicero, A., Perestelo, F., De La Fuente, G., and Trojanowski, J., Appl. Microbiol. Biotechnol., 1996, vol. 45, no. 3, pp. 399–403.
Scherer, M. and Fischer, R., Archiv. Microbiol., 1998, vol. 170, no. 2, pp. 78–84.
Abdel-Raheem, A. and Shearer, C.A., Fungal Diversity, 2002, vol. 11, pp. 1–19.
Wandrey, C., Liese, A., and Kihumbu, D., Org. Proc. Res. Dev., 2000, vol. 4, pp. 285–290.
Couto, S.R. and Harrera, J.L.T., Biotechnol. Adv., 2006, vol. 24, pp. 500–513.
Xu, F., Ind. Biotechnol., 2005, vol. 1, no. 1, pp. 38–50.
Acunzo, D.F. and Galli, C., J. Eur. Biochem., 2003, vol. 270, pp. 3634–3640.
Morozova, O.V., Shumakovich, G.P., Shleev, S.V., and Yaropolov, Y.I., Appl. Biochem. Microbiol., 2007, vol. 43, pp. 523–535.
Coniglio, A., Galli, C., and Gentili, P., J. Mol. Cat. B: Enzymatic, 2008, vol. 50, pp. 40–49.
Mikolasch, A., Niedermeyer, T.H.J., Lalk, M., Witt, S., Seefeld, S., Hammer, E., Schauer, F., Gesell, M., Hessel, S., Julich, W.D., and Lindoquist, U., Chem. Pharm. Bull., 2006, vol. 54, pp. 632–638.
Mikolasch, A., Niedermeyer, T.H.J., Lalk, M., Witt, S., Seefeldt, S., Hammer, E., Schauer, F., Salazar, M.G., Hessel, S., Julich, W.D., and Lindequist, U., Chem. Pharm. Bull., 2007, vol. 55, pp. 412–416.
Mikolasch, A., Hammer, E., Jonas, U., Popowski, K., Stielow, A., and Schaner, F., Tetrahedron, 2002, vol. 58, pp. 7589–7593.
Chaurasia, P.K., Bharati, S.L., Singh, S.K., and Yadava, S., Russ. J. Gen. Chem., 2015, vol. 85, no. 3, pp. 683–685. doi: 10.1134/S1070363215030263
Chaurasia, P.K., Yadav, R.S.S., and Yadava, S., Int. J. Res. Chem. Environ., 2013, vol. 3, no. 1, pp. 188–197.
Chaurasia, P.K., Yadava, S., Bharati, S.L., and Singh, S.K., Synth. Comm., 2014, vol. 44, pp. 2535–2544. doi: 10.1080/00397911.2014.904879
Chaurasia, P.K., Yadav, R.S.S., and Yadava, S., Biochem. Ind. J., 2012, vol. 6, no. 7, pp. 237–242.
Chaurasia, P.K., Yadava, S., Bharati, S.L., and Singh, S.K., Russ. J. Bioorg. Chem., 2014, vol. 40, no. 4, pp. 455–460. doi: 10.1134/S1068162014040025
Chaurasia, P.K., Yadav, A., Yadav, R.S.S., and Yadava, S., J. Chem. Sci., 2013, vol. 125, no. 6, pp. 1395–1403.
Chaurasia, P.K., Yadav, R.S.S., and Yadava, S., Proc. Biochem., 2014, vol. 49, pp. 1647–1655. http://dx.doi.org/10.1016/j.procbio.2014.06.016
Chaurasia, P.K., Yadava, S., Bharati, S.L., and Singh, S.K., Green Chem. Lett. Rev., 2014, vol. 7, no. 1, pp. 100–104. http://dx.doi.org/10.1080/17518253.2014.895869
Leontievsky, A.A., Vares, T., Lankinen, P., Shergill, J.K., Pozdnyakova, N., Myasoedova, N.M., et al., FEMS Microbiol. Lett., 1997, vol. 156, pp. 9–14.
Leontievsky, A., Myasoedova, N., Pozdnyakova, N., and Golovleva, L., FEBS Lett., 1997, vol. 413, pp. 446–448.
Sahay, R., Yadav, R.S.S., and Yadava, S., Appl. Biochem. Biotechnol., 2012, vol. 166, pp. 563–575.
Couto, S.R. and Harrera, J.L.T., Biotechnol. Advan., 2006, vol. 24, pp. 500–513.
Osma, J., Toca-Herrera, J.L., and RodriguezCouto, S., Enz. Res., 2010, article ID918761, pp. 1–8. doi: 10.4061/2010/918761
Brijwani, K., Rigdon, A., and Vadlani, P.V., Enz. Res., 2010, article ID149748, pp. 1–10. doi: 10.4061/2010/149748
Desai, S.S. and Nityanand, C., Asian J. Biotechnol., 2011, vol. 3, no. 2, pp. 98–124.
Coll, M.P., Fernandez-Abalos, J.M., Villanueva, J.R., Santamaria, R., and Perez, P., Appl. Environ. Microbiol., 1993, vol. 59, no. 8, pp. 2607–2613.
Bourbonnais, R. and Paice, M.G., Appl. Microbiol. Biotechnol., 1992, vol. 36, pp. 823–827.
Chefetz, B., Chen, Y., and Hador, Y., Appl. Environ.Microbiol., 1998, vol. 64, pp. 3175–3179.
Potthast, A., Rosenanu, T., Chen, C.-L., and Gratzl, J.S., J. Org. Chem., 1995, vol. 60, pp. 4320–4321.
Fritz-Langhals, E. and Kunath, B., Tetrahedron Lett., 1998, vol. 39, pp. 5955–5956.
Catalogue of Strains (5th ed.), Chandigarh (India): Microbial Type Culture Collection and Gene Bank Institute of Microbial Technology, 2000.
Lowry, O.H., Rosebrough, N.J., Farrand, A.L., and Randall, R.J., J. Biol. Chem., 1951, vol. 193, pp. 265–275.
Laemmli, U.K., Nature, 1970, vol. 227, pp. 680–685.
Polyacrylamide Gel Electrophoresis: Laboratory Techniques, Uppsala (Sweden): Pharmacia, Laboratory Separation Division, 1984.
Weber, K. and Osborn, M., J. Biol. Chem., 1969, vol. 244, pp. 4406–4412.
Schagger, H. and von Jajow, G., Anal. Biochem., 1991, vol. 199, no. 2, pp. 223–231.
Zouri-Mechichi, H., Mechichi, T., Dhouib Sayadi, S., Marrtinez, A.T., and Martinez, M.J., Enz. Microb. Technol., 2006, vol. 39, pp. 141–148.
Chaurasia, P.K., Singh, S.K., and Bharati, S.L., Russ. J. Bioorg. Chem., 2014, vol. 40, no. 3, pp. 288–292. doi: 10.1134/S1068162014020034
Chaurasia, P.K., Bharati, S.L., Singh, S.K., and Yadava, S., Russ. J. Gen. Chem., 2015, vol. 85, no. 1, pp. 173–175. doi: 10.1134/S1070363215010302
Chaurasia, P.K., Bharati, S.L., Yadava, S., and Yadav, R.S.S., Indian J. Biochem. Biophys., 2015, vol. 52, no. 1, pp. 60–67.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sharma, M., Chaurasia, P.K., Yadav, A. et al. Purification and characterization of a thermally stable yellow laccase from Daedalea flavida MTCC-145 with higher catalytic performance towards selective synthesis of substituted benzaldehydes. Russ J Bioorg Chem 42, 59–68 (2016). https://doi.org/10.1134/S1068162016010143
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162016010143