Abstract
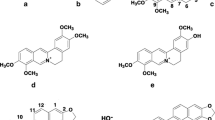
Several 3H-imidazo[4,5-a]acridine derivatives were conveniently synthesized by the reaction of imidazo[4,5-a]acridones in boiling POCl3. The imidazoacridones were obtained by rearrangement of 3H-imidazo[4',5':3,4]benzo[c]isoxazoles in concentrated sulfuric acid containing nitrous acid at room temperature. The structures of all newly synthesized compounds were confirmed by IR, 1H NMR, and mass spectral data. The interactions of 3H-imidazo[4,5-a]acridines with human serum albumin (HSA) were studied by fluorescence spectroscopy. The binding of 3H-imidazo[4,5-a]acridines quenches the HSA fluorescence, revealing a 1: 1 interaction with a binding constant of about 2.34 × 105–3.16 × 106 M–1. A decrease in fluorescence intensity at 339 nm, when excited at 280 nm, is attributed to changes in the environment of the protein fluorophores caused by the presence of the ligand. The differences in interactions of 3H-imidazo[4,5-a]acridines with HSA were observed using spectrofluorimetry technique.
Similar content being viewed by others
References
Bhattacharya, A.A., Grüne, T., and Curry, S., J. Mol. Biol., 2000, vol. 303, pp. 721–732.
Varshney, A., Sen, P., Ahmed, E., Rehan, M., and Subbarao, N., Chirality, 2010, vol. 22, pp. 77–87.
Gulam, J., Zunszain, P.A., Petipas, I., Bhattacharaya, A.A., Otagiri, M., and Curry, S., J. Mol. Biol., 2005, vol. 353, pp. 38–52.
Zaidi, N., Ahmad, E., Rehan, M., Rabbani, G., Ajmal, M. R., Zaidi, Y., et al., J. Phys. Chem. B, 2013, vol. 117, pp. 2595–2604.
Moreno, F., Cortijo, M., and Gonzalez-Jimenez, J., J. Photochem. Photobiol., 1999, vol. 70, pp. 695–700.
Sato, T., Saito, Y., Chikuma, M., Saito, Y., and Nagai, S., Biol. Pharm. Bull., 2008, vol. 31, no. 3, pp. 336–339.
Jones, L.J., Haugland, R.P., and Singer, V.L., Biotechniques, 2003, vol. 34, pp. 850–854.
Belmont, P., Bosson, J., Godet, T., and Tiano, M., Anti-Cancer Agents Med. Chem., 2007, vol. 7, pp. 139–169.
Kamal, A., Srinivas, O., Ramulu, P., Ramesh, G., and Kumar, P.P., Bioorg. Med. Chem. Lett., 2004, vol. 14, pp. 4107–4111.
Demeunynck, M., Expert Opin. Ther. Pat., 2004, vol. 14, pp. 55–70.
Robertson, I.G.C., Palmer, B.D., Officer, M., Siegers, D.J., Paxton, J.W., and Shaw, G.J., Biochem. Pharmacol., 1991, vol. 42, pp. 1879–1884.
Tabarrini, O., Cecchetti, V., Fravolini, A., Nocentini, G., Barzi, A., Sabatini, S., Miao, H., and Sissi, C., J. Heterocycl. Chem., 1999, vol. 42, pp. 2136–2144.
Denny, W.A., Curr. Med. Chem., 2002, vol. 9, pp. 1655–1665.
Goodell, J.R., Madhok, A.A., Hiasab, H., and Ferguson, D.M., Bioorg. Med. Chem., 2006, vol. 14, pp. 5467–5480.
Kukowska-Kaszuba, M. and Dzierzbicka, K., Curr. Med. Chem., 2007, vol. 14, pp. 3079–3104.
Winter, R.W., Kelly, J.X., Smilkstein, M.J., Dodean R., Hinrichs, D., and Riscoe, M.K., Exp. Parasitol., 2008, vol. 118, pp. 487–497.
Smith, J.A., West, R.M., and Allen, M., J. Fluoresc., 2004, vol. 14, pp. 151–171.
Blázquez, M.T., Muñiz, F.M., Sèez, S., Simón, L.M., Alonso, A., Raposo, C., Lithgow, A., Alcázar, V., and Morán, J.R., Heterocycles, 2006, vol. 69, pp. 73–81.
Sadeghian, A., Pordel, M. Safdari, H., Fahmidekar, M.A., and Sadeghian, H., Med. Chem. Res., 2012, vol. 21, pp. 3897–3901.
Rahimizadeh, M., Pordel, M., Bakavoli, M., Bakhtiarpoor, Z., and Orafaie, A., Monatsh. Chem., 2009, vol. 140, pp. 633–638.
Sahraei, R., Pordel, M., Behmadi, H., and Razavi, B., J. Lumin., 2013, vol. 136, pp. 334–338.
Pordel, M., J. Chem. Res., 2012, pp. 595–597.
Daghigh, L.R., Pordel, M., and Davoodnia, A., J. Chem. Res., 2014, vol. 38, pp. 202–207.
Rahmani, Z., Pordel, M., and Davoodnia, A., Bull. Korean Chem. Soc., 2014, vol. 35, pp. 551–556.
Bakavoli, M., Bagherzadeh, G., Vaseghifar, M., Shiri, A., Pordel, M., Mashreghi, M., P. Pordeli, and Araghi, M., Eur. J. Med. Chem., 2010, vol. 45, pp. 647–650.
Rahimizadeh, M., Pordel, M., Bakavoli, M., Rezaeian, Sh., and Sadeghian, A., World. J. Microbiol. Biotechnol., 2010, vol. 26, pp. 317–321.
Pordel, M., Abdollahi, A., and Razavi, B., Russ. J. Bioorg. Chem., 2013, vol. 39, pp. 240–243.
The Chemistry of Heterocyclic Compounds, Part 1, Volume 40: Benzimidazoles and Cogeneric Tricyclic Compounds, Preston, P.N., Ed., John Wiley & Sons, 2009, pp. 87–105.
Tanasescu, I., Bull. Soc. Chim. France, 1927, vol. 41, pp. 528–532.
Rahimizadeh, M., Pordel, M., Bakavoli, M., Eshghi H., and Rezaeian. Sh., Can. J. Chem., 2009, vol. 87, pp. 724–728.
Lakowicz, J.R. and Weber, G., Biochemistry, 1973, vol. 12, pp. 4161–4170.
Gao, H., Lei, L., Liu, J., Qin, K., Chen, X., and Hu, Z., J. Photochem. Photobiol. A, 2004, vol. 167, pp. 213–221.
Lakowicz, J.R., Principles of Fluorescence Spectroscopy, 2nd ed., New York: Kluwer Academic/Plenum Publishers, 1999.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Rahbari, M., Pordel, M. & Chamani, J. Interactions of human serum albumin with bioactive 3H-imidazo[4,5-a]acridines: Insights from fluorescence spectroscopic studies. Russ J Bioorg Chem 42, 36–41 (2016). https://doi.org/10.1134/S1068162016010131
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162016010131