Abstract
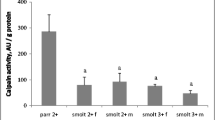
Through the example of the Atlantic salmon Salmo salar L. we provide here the quick details on the universal and specific features of the proteolytic apparatus in the skeletal muscles of fish. Among the numerous muscle tissue proteases, the most comprehensively studied are those which determine the protein degradation levels in the efficiently growing and developing muscles of the salmon juveniles and by this way regulate the protein accumulation rates in muscles and the overall growth processes in the organism, namely, the lysosomal cathepsins B and D and calcium-dependent proteases (calpains). We have detected the age-related differences in the activity of the intracellullar proteases in salmon muscles, which suggest the important role of the proteolysis regulation in growth and the specific role for the certain proteolytic enzymes. For example, we have obtained data indicating the negative correlation between the cathepsin D and calpains activity levels in muscle tissue and the weight gain rate in the salmon of different age groups. The detected positive correlation between the cathepsin B activity and the morphometric indices in juvenile fish is apparently indicative of the key role of this enzyme in the metabolism of proteins of the predominantly nonmyofibrillar nature.
Similar content being viewed by others
Abbreviations
- AMC:
-
aminomethyl coumarine
- CatB, CatD:
-
cathepsins B and D
- DTT:
-
dithiotreitol
- Na-EDTA:
-
sodium ethyl-enediaminetetraacetic acid
- PMSF:
-
phenylmethylsulfonyl fluoride
- SDS:
-
sodium dodecyl sulfate
- TCA:
-
trichloroacetic acid
References
Bond, J.S. and Butler, P.E., Annu. Rev. Biochem., 1987, vol. 56, pp. 333–364.
Nemova, N.N., Vnutrikletochnye proteoliticheskie fermenty u ryb (Intracellular Proteolytic Enzymes in Fish), Petrozavodsk: KarNTs RAN, 1996.
Goll, D.E., Thompson, V.F., Li, H., Wei, W., and Cong, J., Physiol. Rev., 2003, vol. 83, no. 3, pp. 731–801.
Turk, V., Stoka, V., Vasiljeva, O., Renko, M., Sun, T., Turk, B., and Turk, D., Biochim. Biophys. Acta, 2012, vol. 1824, pp. 68–88.
Rock, K.L., Gramm, C., Rothstein, L., Clark, K., Stein, R., Dick, L., Hwang, D., and Goldberg, A.L., Cell, 1994, vol. 78, pp. 761–771.
Dayton, W.R., Goll, D.E., Stromer, M.H., Reville, W.J., Zeece, M.G., and Robson, R.M., in Proteases and Biological Control, Reich, E., Rifkin, D.B., and Shaw, E, Eds., New York: Cold Spring Harbor Lab., 1975, pp. 551–577.
Huang, J. and Forsberg, N.E., Proc. Natl. Acad. Sci. U.S.A., 1998, vol. 95, pp. 12100–12105.
Goll, D.E., Neti, G., Mares, S.W., and Thompson, V.F., J. Anim. Sci., 2008, vol. 86, pp. E19–E35.
Busconi, L., Folco, E.J., Studdert, C., and Sanchez, J.J., Comp. Biochem. Physiol., B, 1992, vol. 102, pp. 303–309.
Seiliez, I., Dias, K., and Cleveland, B.M., Am. J. Physiol. Regul. Integr. Comp. Physiol., 2014, vol. 307, pp. R1330–R1337.
Koodziejska, I. and Smorski, Z.E., J. Food Biochem., 1996, vol. 20, pp. 349–363.
Verrez-Bagnis, V., Ladrat, C., Noelle, J., and Fleurence, J., J. Sci. Food Agric., 2002, vol. 82, pp. 1256–1262.
Mommsen, T.P., Comp. Biochem. Physiol. B, 2004, vol. 139, no. 3, pp. 383–400.
Salem, M., Kenney, B., Rexroad, C., and Yao, J., Comp. Biochem. Physiol. D, 2006, vol. 1, pp. 227–237.
Seiliez, I., Gabillard, J.C., Riflade M., Sadoul, B., Dias, K., Averous, J., Tesseraud, S., Skiba, S., and Panserat, S., Autophagy, 2012, vol. 8, pp. 364–375.
Purintrapiban, J., Wang, M.C., and Forsberg, N.E., Comp. Biochem. Physiol., vol. 136, pp. 393–401.
Mommsen, T.P., Comp. Biochem. Physiol., vol. 129, pp. 207–219.
Johnston, I.A., Bower, N.I., and Macqueen, D.J., J. Exp. Biol, 2011, vol. 214, pp. 1617–1628.
Salem, M., Silverstein, J., Rexroad, C.E., and Yao, J., BMC Genomics, 2007, vol. 8, p. 328.
Overturf, K. and Gaylord, T.G., Comp. Biochem. Physiol., vol. 152, pp. 150–160.
Cleveland, B.M. and Burr, G.S., Aquaculture, 2011, vol. 319, pp. 194–204.
Cleveland, B.M. and Weber, G.M., Gen. Comp. Endocrinol., 2011, vol. 174, pp. 132–142.
Nemova, N.N., Sidorov, V.S., and Ripatti, P.O., Vopr. Ikhtiol., 1980, vol. 20, no. 1, pp. 180–182.
Salmerón, C., García de la Serrana, D., Jiménez-Amilburu, V., Fontanillas, R., Navarro, I., Johnston, I.A., Gutiérrez, J., and Capilla, E., PLoS ONE, 2013, vol. 8, no. 9, p. e75349.
Nemova, N.N., Kaivarainen, E.I., and Bondareva, L.A., Vestnik Mosk. Univ. Ser. 2: Khim., 2000, vol. 41, suppl. 6, pp. 106–108.
Salem, M., Kenney, P.B., Killefer, J., and Nath, J., J. Muscle Foods, 2004, vol. 15, pp. 245–255.
Bahuaud, D., Gaarder, M., Veiseth-Kent, E., and Thomassen, M., Aquaculture, 2010, vol. 310, pp. 213–220.
Gaarder, M., Thomassen, M.S., and Veiseth-Kent, E., Food Chem., 2011, vol. 125, pp. 1091–1096.
Lysenko, L.A., Kantserova, N.P., Ushakova, N.V., and Nemova, N.N., Russ. J. Bioorg. Chem, 2012, vol. 38, pp. 282–290.
Muramoto, M., Yamamoto, Y., and Seki, N., Bull. Jpn. Soc. Sci. Fish., 1989, vol. 55, pp. 917–923.
Salem, M., Yao, J., Rexroad, C., Kenney, B., Semmens, K., Killefer, J., and Nath, J., Comp. Biochem. Physiol. B, 2005, vol. 141, pp. 488–497.
Chéret, R., Delbarre-Ladrat, C., de Lamballerie-Anton, M., and Verrez-Bagnis, V., Food Chem., 2007, vol. 101, pp. 1474–1479.
Yamashita, M. and Konagaya, S., Comp. Biochem. Physiol. B, 1992, vol. 103, no. 4, pp. 999–1003.
Liu, H., Yin, L., Zhang, N., Li, S., and Ma, C., Food Chem., 2008, vol. 110, no. 2, pp. 310–318.
Wang, P.A., Stenvik, J., Larsen, R., Maehre, H., and Olsen, R.L., Comp. Biochem. Physiol. B, 2007, vol. 147, no. 3, pp. 504–511.
Churova, M.V., Meshcheryakova, O.V., Veselov, A.E., and Nemova, N.N., Russ. J. Dev. Biol., 2015, no. 5 (in press).
Ladrat, C., Chaplet, M., Verrez-Bagnis, V., Noel, J., and Fleurence, J., Comp. Biochem. Physiol. B, vol. 125, pp. 83–95.
Cottin, P., Brustis, J.J., Poussard, S., Elamrani, N., Broncard, S., and Ducastaing, A., Acta, 1994, vol. 1223, no. 2, pp. 170–178.
Fukuda, M., Sako, H., Shigeta, T., and Shibata, R., Mar. Biol., 2001, vol. 138, no. 1, pp. 47–55.
Shustov, Yu.A., Baryshev, I.A., and Belyakova, E.N., Biol. Vnutr. Vod, 2012, no. 3, pp. 66–70.
Anson, M.L., J. Gen. Physiol., 1938, vol. 22, pp. 79–89.
Enns, D.L. and Belcastro, A.N., Can. J. Physiol. Pharmacol., 2006, vol. 84, pp. 601–609.
Bradford, M.M., Anal. Biochem., 1976, vol. 72, pp. 248–254.
Arthur, J.S.C. and Mykles, D.L., Calpain. Methods and Protocols, Elce, J.S., Ed., New Jersey: Humana Press, 2000, pp. 109–116.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.A. Lysenko, N.P. Kantserova, M.Yu. Krupnova, A.E. Veselov, N.N. Nemova, 2015, published in Bioorganicheskaya Khimiya, 2015, Vol. 41, No. 6, pp. 717–724.
The article has been adapted from the report presented at the VIIth All-Russian symposium “Proteins and Peptides”, Novosibirsk, 12–17 July, 2015.
Rights and permissions
About this article
Cite this article
Lysenko, L.A., Kantserova, N.P., Krupnova, M.Y. et al. Intracellular protein degradation in the development of the atlantic salmon Salmo salar L.. Russ J Bioorg Chem 41, 645–651 (2015). https://doi.org/10.1134/S1068162015060096
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162015060096