Abstarct
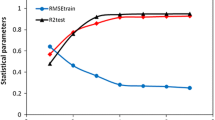
Four stepwise multiple linear regressions (SMLR) and a genetic algorithm (GA) based multiple linear regressions (MLR), together with artificial neural network (ANN) models, were applied for quantitative structure-activity relationship (QSAR) modeling of dissociation constants (K d) of 62 arylsulfonamide (ArSA) derivatives as human carbonic anhydrase II (HCA II) inhibitors. The best subsets of molecular descriptors were selected by SMLR and GA-MLR methods. These selected variables were used to generate MLR and ANN models. The predictability power of models was examined by an external test set and cross validation. In addition, some tests were done to examine other aspects of the models. The results show that for certain purposes GA-MLR is better than SMLR and for others, ANN overcomes MLR models.
Similar content being viewed by others
References
Maestrelli, F., Mura, P., Casini, A., Mincione, F., Scozzafava, A., and Supuran, C.T., J. Pharm. Sci., 2002, vol. 91, no. 10, pp. 2211–2219.
Melagraki, G., Afantitis, A., Sarimveis, H., Igglessi-Markopoulou, O., and Supuran, C.T., Bioorgan. Med. Chem., 2006, vol. 14, no. 4, pp. 1108–1114.
Agrawal, V.K., Banerji, M., Gupta, M., Singh, J., Khadikar, P.V., and Supuran, C.T., Eur. J. Med. Chem., 2005, vol. 40, no. 10, pp. 1002–1012.
Winum, J.Y., Scozzafava, A., Montero, J.L., and Supuran, C.T., Med. Res. Rev., 2005, vol. 25, no. 2, 186–228.
Zimmerman, S., Innocenti, A., Casini, A., Ferry, J.G., Scozzafava, A., and Supuran, C.T., Bioorg. Med. Chem. Lett., 2004, vol. 14, no. 24, pp. 6001–6006.
Chazalette, C., Masereel, B., Rolin, S., Thiry, A., Scozzafava, A., Innocenti, A., and Supuran, C.T., Bioorg. Med. Chem. Lett., 2004, vol. 14, no. 23, pp. 5781–5786.
Rusconi, S., Innocenti, A., Vullo, D., Mastrolorenzo, A., Scozzafava, A., and Supuran, C.T., Bioorg. Med. Chem. Lett., 2004, vol. 14, no. 23, pp. 5763–5767.
Krishnamurthy, V.M., Kaufman, G.K., Urbach, A.R., Gitlin, I., Gudiksen, K.L., Weibel, D.B., and Whitesides, G.M., Chem. Rev., 2008, vol. 108, no. 3, pp. 946–1051.
Dashtbozorgi, Z. and Golmohammadi, H., J. Sep. Sci., 2010, vol. 33, nos. 23–24, pp. 3800–3810.
Dashtbozorgi, Z. and Golmohammadi, H., Eur. J. Med. Chem., 2010, vol. 45, no. 6, 2182–2190.
Hansch, C., Hoekman, D., Leo, A., Zhang, L.T., and Li, P., Toxicol. Lett., 1995, vol. 79, nos. 1–3, pp. 45–53.
Krogsgaard-Larsen, P., Frolund, B., and Liljefors, T., Chem. Rec., 2002, vol. 2, no. 6, pp. 419–430.
Consonni, V. and Todeschini, R., Handbook of Molecular Descriptors, Weinheim: Wiley-VCH, 2000.
Deeb, O., Hemmateenejad, B., Jaber, A., Garduno-Juarez, R., and Miri, R., Chemosphere, 2007, vol. 67, no. 11, pp. 2122–2130.
Mandloi, D., Joshi, S., Khadikar, P.V., and Khosla, N., Bioorg. Med. Chem. Lett., 2005, vol. 15, no. 2, pp. 405–411.
Johnson, T., Khan, I.A., Avery, M.A., Grant, J., and Meshnick, S.R., Antimicrob. Agents Ch., 1998, vol. 42, no. 6, pp. 1454–1458.
Thakur, A., Thakur, M., Khadikar, P.V., Supuran, C.T., and Sudele, P., Bioorgan. Med. Chem., 2004, vol. 12, no. 4, pp. 789–793.
Hatami, T., Rahimi, M., Daraei, H., Heidaryan, E., Alsairafi, A.A., and Korean, J., Chem. Eng., 2012, vol. 29, no. 5, pp. 657–667.
Daraei, H., Irandoust, M., Ghasemi, J.B., and Kurdian, A.R., J. Incl. Phenom. Macro., 2012, vol. 72, nos. 3–4, pp. 423–435.
Depczynski, U., Frost, V.J., and Molt, K., Anal. Chim. Acta, 2000, vol. 420, no. 2, pp. 217–227.
Jouanrimbaud, D., Massart, D.L., Leardi, R., and Denoord, O.E., Anal. Chem., 1995, vol. 67, no. 23, pp. 4295–4301.
Maleki, A., Daraei, H., Alaei, L., Abasi, L., and Izadi, A., J. Chem. Soc. Pak., 2012, vol. 34, no. 5, pp. 1056–1069.
Rocha, Pita S.S., Albuquerque, M.G., Rodrigues, C.R., Castro, H.C., and Hopfinger, A.J., Chem. Biol. Drug Design, 2012, vol. 79, no. 5, pp. 740–748.
Magee P.S., Quantitative Structure-Activity Relationships, 1990, vol. 9, no. 3, pp. 202–215.
Jalali-Heravi, M., Asadollahi-Baboli, M., and Shahbazikhah, P., Eur. J. Med. Chem., 2008, vol. 43, no. 3, pp. 548–556.
Nieto, M., Alovero, F., Manzo, R., and Mazzieri, M.R., Eur. J. Med. Chem., 2005, vol. 40, no. 4, pp. 361–369.
Rastija, V. and Medić-Šarić, M., Eur. J. Med. Chem., 2009, vol. 44, no. 1, pp. 400–408.
Kumar, P., Singh, P., and Singh, J.P., Int. J. Res. Pharm. Biomed. Sci., 2012, vol. 3, no. 1, pp. 65–72.
Glasstone, S., Thermodynamics for Chemists, Huntington: R.E. Krieger Pub. Co., 1972.
Ivanciuc, O., Ivanciuc, T., Klein, D.J., Seitz, W.A., and Balaban, A.T., J.Chem. Inf. Comput. Sci., 2001, vol. 41, no. 3, pp. 536–549.
Dong, H.W. and Guo, X.F., Match-Commun. Math. Co., 2010, vol. 63, no. 3, pp. 799–812.
Rücker, C., Scarsi, M., and Meringer, M., Bioorgan. Med. Chem., 2006, vol. 14, no. 15, pp. 5178–5195.
Hemalatha, R., Soni, L., Gupta, A., and Kaskhedikar, S., E-Journal of Chemistry, 1900, vol. 1, no. 5, pp. 243–250.
Chen, L., Yao, J., and Yang, J., Acta Pharmacol. Sin., 2005, vol. 26, no. 11, pp. 1322–1333.
Afantitis, A., Melagraki, G., Sarimveis, H., Koutentis, P.A., Markopoulos, J., and Igglessi-Markopoulou, O., Polymer, 2006, vol. 47, no. 9, pp. 3240–3248.
Rucker, C., Rucker, G., and Meringer, M., J. Chem. Inf. Model., 2007, vol. 47, no. 6, pp. 2345–2357.
Karki, R.G. and Kulkarni, V.M., Bioorgan. Med. Chem., 2001, vol. 9, no. 12, pp. 3153–3160.
Kovarich, S., Papa, E., and Gramatica, P., J. Hazard. Mater., 2011, vol. 190, nos. 1–3, pp. 106–112.
Topliss, J.G. and Edwards, R.P., J. Med. Chem., 1979, vol. 22, no. 10, pp. 1238–1244.
Su, L., Li, L., Li, Y., Zhang, X., Huang, X., and Zhai, H., Med. Chem. Res., 2011, pp. 1–18.
Asadollahi, T., Dadfarnia, S., Shabani, A.M.H., Ghasemi, J.B., and Sarkhosh, M., Molecules, 2011, vol. 16, no. 3, pp. 1928–1955.
Li, F., Wu, H.F., Li, L.Z., Li, X.H., Zhao, J.M., and Peijnenburg, W.J.G.M., Ecotox. Environ. Saf., 2012, vol. 80, pp. 273–279.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Maleki, A., Daraei, H., Alaei, L. et al. Comparison of QSAR models based on combinations of genetic algorithm, stepwise multiple linear regression, and artificial neural network methods to predict K d of some derivatives of aromatic sulfonamides as carbonic anhydrase II inhibitors. Russ J Bioorg Chem 40, 61–75 (2014). https://doi.org/10.1134/S106816201306006X
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816201306006X