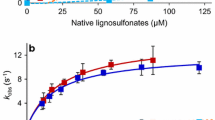
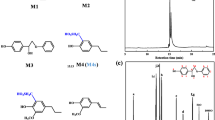
Abstract
The published information on the use of enzymes belonging to a large family of peroxidases of plant and fungal origin as the catalysts of lignin and its model compound oxidation by hydrogen peroxide are reviewed. The structures and the mechanism of the catalytic action of these enzymes are comparatively considered. The enzymes have similar structures; however, the enzymes of plant origin have higher stabilities and pH optima. It was concluded that further studies of the effect of the functional nature, polymolecular properties, and regularities of the redox conversions during the catalytic oxidation of plant lignins by plant peroxidases are promising.
Similar content being viewed by others
References
Andreeva, V.A., Ferment peroksidaza: uchastie v zashchitnom mekhanizme rastenii (Enzyme Peroxidase: Involvement in Defensive Mechanisms of Plants), Moscow, 1988.
Gazaryan, I.G., Khushpul’yan, D.M., and Tishkov, V.I., Usp. Biol. Khim., 2006, vol. 46, pp. 303–322.
Young, R.A. and Akhtar, M., Environmentally Friendly Technologies for Paper Industry, Canada: Wiley, 1998.
Have, R. and Teunissen, P.J., Chem. Rev., 2001, no. 101, pp. 3397–3413.
Levit, M.N. and Shkrob, A.M., Bioorg. Khim., 1992, vol. 18, no. 3, pp. 309–345.
Bekker, E.G., Isolation, Properties, and Major Consistent Patterns of Action of Lignolytic Enzymes (Laccases, Ligninases, and Mn-Peroxidases), Cand. Sci. (Chem.), Dissertation, Moscow, 1993.
Bolobova, A.V., Askadskii, A.A., Kondratenko, V.I., and Rabinovich, M.L., Teoreticheskie osnovy biotekhnologii drevesnykh kompozitov (Theoretical Basics of Biotechnology of Woody Composites), Book II: Fermenty, modeli, protsessy (Enzymes, Models, and Processes), Moscow, 2002.
Shimada, M. and Higuchi, L., in Wood and Cellulosic Chemistry, Hon, D.N.-S. and Shiraishi, N., Eds., New York: Mercek Dekker, 1991, pp. 525–591.
Strtel’skii, V.A., Beigel’man, A.V., and Chupka, E.I., Khim. Prir. Soedin., 1982, no. 1, pp. 109–112.
Strel’skii, V.A., Peroxidase Oxidation of Lignin and Its Model Compounds, Cand. Sci. (Chem.) Dissertation, Leningrad, 1986.
Hewson, W.D. and Dunford, H.B., J. Biol. Chem., 1976, vol. 251, no. 19, pp. 6036–6042.
Thompson, D., Norbeck, K., Olsson, L., and Constantin-Teodosiu, D., J. Biol. Chem., 1989, vol. 264, no. 2, pp. 1016–1021.
Banci, L., Bertini, I., Bini, T., et al., Biochemistry, 1993, vol. 32, no. 22, pp. 5825–5831.
Smith, A.T. and Veitch, N.C., Curr. Opin. Chem. Biol., 1998, vol. 2, pp. 269–278.
Kuila, D., Tien, M., Fee, J.A., and Ondrias, M.R., Biochemistry, 1985, vol. 24, pp. 3394–3397.
Andersson, L.A., Renganathan, V., Chiu, A., et al., J. Biol. Chem., 1985, vol. 260, no. 10, pp. 6080–6087.
Andersson, L.A., Renganathan, V., Loehr, T.M., et al., Biochemistry, 1987, vol. 26, no. 8, pp. 2258–2263.
Henricsen, A., Smith, A.T., and Gajhede, M., J. Biol. Chem., 1999, vol. 274, pp. 35005–35011.
Montellano, O., Annu. Rev. Pharmacol. Toxicol., 1992, vol. 32, pp. 89–107.
Rodrigues-Lopez, J.N., Gilabert, M.A., Tundela, J., and Thorneley, R.N., Biochemistry, 2000, vol. 39, pp. 13201–13209.
Dunford, H.B. and Adeniran, A.J., Arch. Biochem. Biophys., 1986, vol. 251, pp. 536–542.
Van Haandel, M.J., Classens, M.M., and Van der Hount, N., Biochim. Biophys. Acta, 1999, vol. 1435, pp. 22–29.
Schneegab, I., Hofrichter, M., Scheibner, K., and Fritsche, W., Appl. Microbiol. Biotechnol., 1997, vol. 48, pp. 602–605.
Crestini, C., DAnnibale, A., Sermanni, G.G., and Saladino, R., Bioorg. Med. Chem., 2000, vol. 8, no. 2, pp. 433–438.
Gelpke, M.D.S., Youngs, H.L., and Gold, M.N., Eur. J. Biochem., 2000, vol. 267, pp. 7038–7054.
Bockle, B., Martinez, M.J., Guillen, F., and Martinez, A.T., Appl. Environ. Microbiol., 1999, vol. 65, no. 3, pp. 923–928.
Marbach, I., Harel, E., and Mayer, A.M., Phytochemistry, 1985, vol. 24, pp. 2559–2561.
Xu, F., J. Biol. Chem., 1997, vol. 272, no. 2, pp. 924–928.
Tien, M. and Kirk, T.K., Proc. Natl. Acad. Sci. USA, 1984, vol. 81, no. 8, pp. 2280–2284.
Kuila, D., Tien, M., Fee, J.A., and Ondrias, M.R., Biochemistry, 1985, vol. 24, no. 14, pp. 3394–3397.
Gold, M.H., Wariishi, H., and Valli, K., ACS Symp. Ser., 1989, vol. 389, pp. 127–140.
La Mar, G.N., Thanabal, V., and De Ropp, J.S., Biochemistry, 1989, vol. 28, no. 4, p. 1934.
Godfrey, B.J., Mayfield, M.B., Brown, J.A., and Gold, M.H., Gene, 1990, vol. 93, no. 1, pp. 119–124.
Renganathan, V. and Gold, M.H., Biochemistry, 1986, vol. 25, no. 7, pp. 1626–1631.
Dure, L. and Cormier, M.J., J. Biol. Chem., 1964, vol. 239, pp. 2351–2359.
Tien, M., Kirk, T., Bull, C., and Fee, J.A., J. Biol. Chem., 1986, vol. 261, no. 4, pp. 1687–1693.
Glenn, J.K. and Gold, M.H., Arch. Biochem. Biophys., 1985, vol. 242, no. 2, pp. 329–341.
Kersten, P.J., Kalyanaraman, B., Hammel, K.E., Reinhammar, B., and Kirk, T.K., Biochem. J., 1990, vol. 268, no. 2, pp. 475–480.
Schoemaker, H.E., Harvey, P.J., Bowen, R.M., and Palmer, J.M., FEBS Lett., 1985, vol. 183, no. 1, pp. 7–12.
Band, L., Ciofi-Baffoni, S., and Tien, M., FEBS Lett., 1999, vol. 38, no. 10, pp. 3205–3210.
Kirk, T.K. and Farrel, R.L., Annu. Rev. Microbiol., 1987, pp. 125–131.
Cardwell, E.S. and Steellink, C., Biochem. Biophys. Acta, 1969, pp. 105–109.
Loung, M. and Steellink, C., Phytochemistry, 1973, vol. 12, pp. 2152–2157.
Khindaria, A., Grover, T.A., and Aust, S.D., Biochemistry, 1995, vol. 34, pp. 6020–6025.
Koduri, R.S. and Tien, M., J. Biol. Chem., 1995, vol. 270, no. 38, pp. 22254–22258.
Laszlo, J.A. and Compton, D.L., J. Mol. Cat. B, 2002, vol. 18, pp. 109–120.
Ward, G., Hadar, Y., and Dosoretz, C.G., Enzyme Microb. Technol., 2001, vol. 29, no. 1, pp. 34–41.
Outgenoeg, G., Dirksen, E., Ingemann, S., and Hilhorst, R., J. Biol. Chem., 2002, vol. 277, no. 24, pp. 21332–21340.
Tompson, D., Norbeck, K., Olsson, L., and Constantin-Teodosiu, D., J. Biol. Chem., 1989, vol. 264, no. 2, pp. 1016–1021.
Holmgren, A., Biochemical Control Aspects in Lignin Polymerization, Doctoral Thesis, Stockholm: Royal Institute of Technology, 2008.
Kirk, T.K., Biochemistry and Genetics of Cellulose Degradation, Academic Press, 1988, pp. 315–332.
Hwang, R.H., Kennedy, J.F., and Melo, E.H.M., Carbohydrate Polymers, 1989, vol. 10, no. 1, pp. 15–30.
Kurek, B., Monties, B., and Odier, E., Enzyme Microb. Technol., 1990, vol. 12, no. 10, pp. 771–777.
Hammel, K.E. and Moen, M.A., Enzyme Microb. Technol., 1991, vol. 13, no. 1, pp. 15–18.
Howes, B.D., Feis, A., Raimondi, L., and Smulevich, G., J. Biol. Chem., 2001, vol. 276, no. 44, pp. 40704–40711.
Klapper, M. and Hackett, D.P., J. Biol. Chem., 1963, vol. 238, no. 11, pp. 3736–3742.
Ugarova, N.N., Lebedeva, O.V., and Savitskii, A.P., Peroksidaznyi kataliz i ego primenenie (Peroxidase Catalysis and Its Application), Moscow, 1981.
Neilsen, K.L., Indiani, G., Henriksen, A., et al., Biochemistry, 2001, vol. 40, pp. 11013–11021.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.A. Eisenstadt, K.G. Bogolitsyn, 2010, published in Khimiya Rastitel’nogo Syr’ya, 2009, No. 2, pp. 5–18.
Rights and permissions
About this article
Cite this article
Eisenstadt, M.A., Bogolitsyn, K.G. Peroxidase oxidation of lignin and its model compounds. Russ J Bioorg Chem 36, 802–815 (2010). https://doi.org/10.1134/S1068162010070034
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162010070034