Abstract
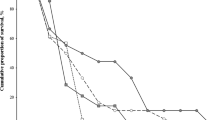
Populations from different environmental habitats indicate variation in life history traits and these differences are mostly related to longevity, age and size at maturity. In this study, age structure, longevity, survivorship and sexual size dimorphism of Near Eastern fire salamander (Salamandra infraimmaculata) were assessed by means of skeletochronological analysis. Maximum lifespan was recorded to be twelve years in females and eleven years in males and age at maturity was estimated as three and four years for both sexes. Females had significantly larger SVL than that of males, while age structure and mean age did not differ between sexes. Age and body size were positively correlated with each other for both females and males. Since the populations of the Near Eastern fire salamander in Turkey are in decline, the present study which provides preliminary data on life history traits of this species could be helpful for future biological studies.
Similar content being viewed by others
References
Papenfuss, T., Disi, A., Rastegar-Pouyani, N., et al., Salamandra infraimmaculata, in The IUCN Red List of Threatened Species, 2009: E.T59466a11927871. http://Dx.Doi.Org/10.2305/Iucn.Uk.2009.Rlts.T5946 6a11927871.En. Accessed June 16, 2016.
Blank, L. and Blaustein, L., Using ecological niche modeling to predict the distributions of two endangered amphibian species in aquatic breeding site, Hydrobiologia, 2012, vol. 693, no. 1, pp. 157–167.
The IUCN Red List of Threatened Species, Version 2014.3. Gland, Switzerland: IUCN, 2016.
Altunisik, A. and Özdemir, N., Life history traits in Bufotes variabilis (Pallas, 1769) from 2 different altitudes in Turkey, Turk. J. Zool., 2015, vol. 39, no. 1, pp. 153–159.
Castanet, J., Francillon-Vieillot, H., and Bruce, R.C., Age estimation in desmognathine salamanders assessed by skeletochronology, Herpetologica, 1996, vol. 52, no. 2, pp. 160–171.
Paton, D., Jurranz, A., Sequeros, E., et al., Seasonal age and sex structure of Rana perezi assessed by skeletochronology, J. Herpetol., 1991, vol. 25, no. 4, pp. 389–394.
Krebs, C.J., Ecology, San Francisco: Benjamin Cummings, 2001.
Eden, C.J., Whiteman, H.H., Duobinis-Gray, L., and Wissinger, S.A., Accuracy assessment of skeletochronology in the Arizona tiger salamander (Ambystoma tigrinum nebulosum), Copeia, 2007, vol. 2007, no. 2, pp. 471–477.
Caetano, M.H., Use and results of skeletochronology in some urodeles (Triturus marmoratus, Latreille 1800 and Triturus boscai, Lataste 1879), Ann. Sci. Nat. Zool., 1990, vol. 11, no. 4, pp. 197–199.
Castanet, J. and Smirina, E.M., Introduction to the skeletochronological method in amphibians and reptiles, Ann. Sci. Nat. Zool., 1990, vol. 1990, no. 13, pp. 191–196.
Özdemir, N., Altunisik, A., Ergül, T., et al., Variation in body size and age structure among three Turkish populations of the tree frog Hyla arborea, Amphibia Reptilia, 2012, vol. 33, no.1, pp. 25–35.
Altunisik, A. and Özdemir, N., Body size and age structure of a highland population of Hyla orientalis Bedriaga, 1890, in Northern Turkey, Herpetozoa, 2013, vol. 26, nos. 1–2, pp. 49–55.
Ergül Kalayci, T., Özdemir, N., Altunisik, A. and Gül, S., The effect of altitude, latitude and climatic variables on life-history traits of male Hyla savignyi (Audouin, 1827) from Anatolia (Turkey): A skeletochronological study (Anura: Hylidae), Herpetozoa, 2015, vol. 28, nos. 1–2, pp. 55–62.
Sinsch, U., Pelster B. and Ludwig G. Large-scale variation of size-and age-related life-history traits in the common frog: A sensitive test case for macroecological rules, J. Zool., 2015, vol. 297, no. 1, pp. 32–43.
Warburg, M. R., The phenology of a rare salamander (Salamandra infraimmaculata) in a population breeding under unpredictable conditions: A 25-year study, Acta Herpetol., 2007, vol. 2, no. 2, pp. 147–157.
Segev, O., Hill, N., Templeton, A. R. and Blaustein, L., Population size, structure and phenology of an endangered salamander at temporary and permanent breeding sites, J. Nat. Conserv., 2010, vol. 2010, no. 18, pp. 189–195.
Warburg, M.R., Age and size at metamorphosis of halfsib Salamandra infraimmaculata larvae born in the laboratory and raised singly under three different food regimes, Belg. J. Zool., 2009, vol. 139, no. 2, pp. 156–165.
Goldberg, T., Nevo, E. and Degani, G., Genetic diversity and different ecological conditions in Salamandra infraimmaculata larvae from various breeding sites, Anim. Biol., 2011, vol. 2, no. 2, pp. 37–49.
Warburg, M.R., Age and size at metamorphosis of Salamandra infraimmaculata larvae born in the laboratory and raised under different density regimes with food ad libitum, Salamandra, 2012, vol. 48, no. 3, pp. 157–165.
Warburg, M.R., Population ecology, breeding activity, longevity, and reproductive strategies of Salamandra salamandra during an 18-year-long study of an isolated population on Mt. Carmel, Israel, Mertensiella, 1994, vol. 1994, no. 4, pp. 399–421.
Warburg, M. R., Longevity in Salamandra infraimmaculata from Israel with a partial review on other salamanders, Salamandra, 2007, vol. 43, no. 1, pp. 21–34.
Altunisik, A., Sexual size and shape dimorphism in the Near Eastern fire salamander, Salamandra infraimmaculata (Caudata: Salamandridae), Anim. Biol., 2017, vol. 67, no. 1, pp. 29–40.
http://www.mgm.gov.tr. Accessed June 03, 2016.
Lovich, J. E. and Gibbons, J.W., A review of techniques for quantifying sexual size dimorphism. Growth Dev. Aging, 1992, vol. 56, no. 4, 269–281.
Olgun, K., Miaud, C. and Gautier, P., Age, growth, and survivorship in the viviparous salamander Mertensiella luschani from Southwestern Turkey, Can. J. Zool., 2001, vol. 79, no. 9, pp.1559–1567.
Seber, G.A.F., The Estimation of Animal Abundance and Related Parameters, London: Griffin, 1973.
Vitt, L.J. and Caldwell, J.P., Herpetology, an Introductory Biology of Amphibians and Reptiles, Burlington, MA: Academic, 2009.
Reinhard, S., Renner, S. and Kupfer, A., Age and fecundity in Salamandra algira (Caudata: Salamandridae), Salamandra, 2015, vol. 51, no. 1, pp. 19–24.
Kozlowski, J. and Uchmanski, J., Optimal individual growth and reproduction in perennial species with indeterminate growth, Evol. Biol., 1987, vol. 1, pp. 214–230.
Rebelo, R. and Caetano, M. H., Use of skeletochronological method for ecodemographical studies on Salamandra salamandra gallaica from Portugal, in Scientia Herpetologica, Llorente, G.A., Montori, A., Santos, X., and Carretero, M.A., Eds., Barcelona: Asociación Herpetológica Española, 1995, pp. 135–140.
Miaud, C., Andreone, F., Riberon, A., et al., Differences in age, size at maturity and gestation duration among two neighbouring populations of the Alpine salamander Salamandra lanzai, J. Zool., 2001, vol. 254, pp. 251–260.
Kutrup, B., Bülbül, U. and Yilmaz, N., Age structure in two populations of Triturus vittatus ophryticus at different altitudes, Amphibia Reptilia, 2005, vol. 26, no. 1, pp. 49–54.
Altunisik, A., Ergül, T., Gül, Ç., et al., A skeletochronological study of the smooth newt Lissotriton vulgaris (Amphibia: Urodela) from an island and a mainland population in Turkey, Ital. J. Zool., 2014, vol. 81, no. 3, pp. 381–388.
Miaud, C., Guyetant, R. and Faber, H., Age, size and growth of the Alpine newt, Triturus alpestris (Urodela, Salamandridae), at high altitude and a review of lifehistory trait variation throughout its range, Herpetologica, 2000, vol. 56, no. 2, pp. 135–144.
Gümüs Özcan, Ç. and Üzüm, N., Body size and age in three populations of the northern banded newt Ommatotriton ophryticus (Berthold, 1846) from Turkey, Turk. J. Zool., 2015, vol. 39, no. 4, pp. 672–679.
Shine, R., Sexual selection and sexual dimorphism in the Amphibia, Copeia, 1979, vol. 1979, no. 2, pp. 297–306.
Kupfer, A., Sexual size dimorphism in amphibians: An overview, in Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism, Fairbairn, D.J., Blanckenhorn, W.U., and Szèkely, T., Eds., New York: Oxford Univ. Press, 2007, pp. 50–59.
Reinhard, S., Renner, S. and Kupfer, A., Sexual dimorphism and age of Mediterranean salamanders, Zoology, 2015, vol. 118, no. 1, pp. 19–26.
Malmgren, J.C. and Thollesson, M., Sexual size and shape dimorphism in two species of newts, Triturus cristatus and T. vulgaris (Caudata: Salamandridae), J. Zool., 1999, vol. 249, no. 2, pp. 127–136.
Olgun, K., Üzüm, N., Avci A. and Miaud, C., Age, size and growth of the southern crested newt Triturus karelinii (Strauch 1870) in a population from Bozdag (Western Turkey), Amphibia Reptilia, 2005, vol. 26, pp. 223–230.
Bovero, S., Sotgiu, G., Castellano, S. and Giacoma, C., Age and sexual dimorphism in a population of Euproctus platycephalus (Caudata: Salamandridae) from Sardinia, Copeia, 2003, vol. 2003, no. 1, pp. 149–154.
Üzüm, N., A skeletochronological study of age, growth and longevity in apopulation of the Caucasian Salamander, Mertensiella caucasica (Waga 1876) (Caudata: Salamandridae) from Turkey, North-West. J. Zool., 2009, vol. 5, no.1, pp. 74–84.
Shine, R., The evolution of large body size in females: A critique of Darwin’s fecundity advantage model, Am. Nat., 1988, vol. 131, no.1, pp. 124–131.
Blanckenhorn, W.U., Behavioural causes and consequences of sexual sizedimorphism, Ethology, 2005, vol. 111, no. 11, pp. 977–1016.
Liao, W.B., Liu, W.C. and Merila, J., Andrew meets Rensch: Sexual size dimorphism and the inverse of Rensch’s rule in Andrew’s toad (Bufo andrewsi), Oecologia, 2015, vol. 177, pp. 389–399.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Altunışık, A. Age, Survivorship and Life Expectancy in Near Eastern Fire Salamander, Salamandra infraimmaculata (Caudata: Salamandridae). Russ J Ecol 49, 166–171 (2018). https://doi.org/10.1134/S1067413618020029
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1067413618020029