Abstract
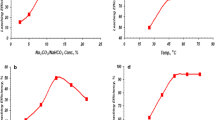
An alternative procedure was developed for selective leaching of uranium from carbonate-rich black shale of southwestern Sinai (0.16% U3O8). Uranium was present in the rock sample in the hexavalent state. This method is based on using a mixed citric acid/calcium citrate reagent so as to leave the carbonate, vanadium, and copper almost intact for the later processing. The influence of the concentration of citric acid/Ca citrate, molar ratio, grain size, pH of the leach liquor solutions, solid-to-liquid ratio, leaching time, and temperature was investigated in order to find optimum conditions. From the leach liquor, uranium can be recovered by addition of isopropyl alcohol.



Similar content being viewed by others
Change history
26 April 2021
An Erratum to this paper has been published: https://doi.org/10.1134/S1066362221020168
REFERENCES
Chang, P., Kim, K.W., Yoshida, S., and Kim, S.Y., J. Environ. Geochem. Health, 2005, vol. 27, pp. 529–538.
Riegel, M., Tokmachev, M., and Hoell, W.H., J. React. Funct. Polym., 2008, vol. 68, pp. 1072–1080.
Singh, D.K., Hareendran, K.N., Sreenivas, T., Kain, V, and Dey, G.K., J. Hydrol., 2017, vol. 171, pp. 228–235.
Kunin, R., Yaradleg, P., Larterra, T., and Old Bridge, N.J., Patent US 4606894, 1986.
Forward, F.A., Halpern, J., and Couver, V., Patent US 2727806, 1955.
Hamza, M.F., El-Aassy, I.E., and Guibalb, E., J. Min. Eng., 2019, vol. 133, pp. 138–148.
. Yantasee, W., Fryxell, G.E., Pattamakomsan, K., Sangvanich, T., Wiacek, R.J., Busche, B., Addleman, R.S., Timchalk, C., Ngamcherdtrakul, W., and Siriwon, N., J. Hazard. Mater., 2019, vol. 366, pp. 677–683.
Alter, I., in Int. Conf. on Peaceful Uses of Atomic Energy, Geneva, 1958, vol. 3.
Kennedy, R.H., Proc. Panel, Vienna: IAEA, 1967.
Ketzinel, Z., Yakir, D., Rosenberg, J., Shashua, J., Hasid, M., and Volkman, Y., Proc. San Paulo Symp., IAEA-SM-135/12, Vienna, 1971, IAEA-SM-135/12, Vienna.
Nettleton, K.C.A., Nikoloski, A.N., and Da Costa, M., J. Hydrol., 2015, vol. 157, pp. 270–279.
Singh, D.K., Hareendran, K.N., Sreenivas, T., Kain, V., and Dey, G.K., J. Hydrol., 2017, vol. 171, pp. 228–235.
Li, M., Huang, C.M., Zhang, X.W., Gao, F.Y., Wu, X.Y., Fang, Q., Tan, W.F., and Zhang, D., J. Hydrol., 2018, vol. 180, pp. 201–209.
Lu, B.Q., J. Hazard. Mater., 2018, vol. 343, pp. 255–265.
Thunnaes, A., Brown, E.A., Rabbits, A.T., Simard, R., and Ottawa, H.I., Patent US 2992887, 1961.
El Hazek, N.M.T., Milad, N.E., and Hussein, E.M., Bull. Fac. Sci., Ain Shams Univ., 1980, vol. 22.
Glusker, J.P., Acc. Chem. Res., 1980, vol. 13, no. 3, pp. 45–52.
Bashir, S., Hawaz, H., and Bahatti, T.M., Int. J. Agricult. Biol., 2000, vol. 2, nos. 1–2.
Hussein, E.M., PhD Thesis, Fac. Sci., Ain Shams Univ., 1980.
Krumm, H.E., S. Afr. J. Sci., 1953, vol. 49.
El Hazek, N.M.T., Milad, N.E., and Hussein, E.M., Ain Shams Sci. Bull., 1980, no. 22.
Hussein, E.M., Patent EG 3384 17514, Jan. 18, 1984
Farag, A.B., Bakry, A.R., Abdelfattah, N.A., and Elwy, A.M., Int. J. Adv. Res., 2015, vol. 3, no. 5, pp. 32–41.
Bakry, A.R., and El Hady, S.M., Chem. Technol. Indian J., 2015, vol. 10, no. 6, pp. 266–275.
Mustafa, V., and Ghaly, E.L., J. Chem. Eng. Data, 1964, vol. 9, no. 4, pp. 557–567.
El-Kammar, A., J. Arab. Geo-Frontiers, 2014, vol. 1, pp. 1–34.
Davies, W., and Gray, W., TAL J., 1964, vol. 11, no. 8, pp. 1203–1211.
Shapiro, L., and Brannock, W.W.U.S. Geol. Surv. Bull., 1962, vol. 114A, pp. 114–132.
Newman, L., Adin, A., and Klotz, P., Inorg. Chem., 1970, vol. 9, p. 2499 .
Skoog, T.M., Special Publication of Chemical Society of America, 1992, vol. 17, p. 447.
Dodge, C.J. and Francis, A.I., Environ. Sci. Technol., 1997, vol. 31, pp. 62–67.
Bhatti, T.M., Khalid, A.M., and Malik, K.A., Biochem. Biophys., 1997, vol. 2, pp. 215–218.
Kantar, C., and Honeyman, B.D., J. Environ. Eng., 2012, vol. 132, no. 2, pp. 247–255.
Ohyoshi, E., Oda, J., and Ohyoshi, A., Bull. Chem. Soc. Jpn., 1975, vol. 48, no. 1, pp. 7–9.
Heitner, C., and Botelsky, M., Bull. Soc. Chim. Fr., 1954, vol. 21.
Neuman, W.F., and Havil, J.R., USAEC Report, 1949, no. 2829.
Rajan, K.S., and Martell, A.E., Report of Illinois Inst. of Technology, Chicago, 1964, no. 11-1, p. 1020.
Traut, D.E., El-Hazek, N.M.T., Palmer, G.R., and Nicholes, I.L., U.S. RI-8328, U.S. Bureau of Mines, 1979, vol. 13.
Krivovichev, S.V. and Plasil, J., Mineralogy and Crystallography of Uranium, Winnipeg: Mineralogical Association of Canada, 2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Bakry, A.R. An Alternative Procedure for Selective Leaching of Uranium from Carbonate-Rich Black Shale, Um-Bogma Formation, El-Allouga, Southwestern Sinai. Radiochemistry 63, 87–95 (2021). https://doi.org/10.1134/S1066362221010136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362221010136