Abstract
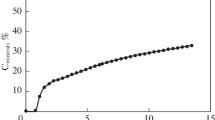
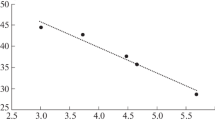
Transformation of water-soluble organic substances entering mineral horizons of podzol from the forest litter was studied in a model column experiment. The concentration of organic matter in the solution after its interaction with the E horizon increased by 10–44% due to the release of native soluble organic matter. This was accompanied by changes in the quality of water-soluble organic matter: the portion of hydrophobic components increased, the coefficients of extinction at 254–280 nm decreased, and the portion of low-molecular-weight compounds slightly decreased with a corresponding increase in the portion of high-molecular-weight compounds. Upon the interaction of water-soluble organic substances with the E horizon, the pH values first decreased and then slightly increased, which could be due to sequential desorption of organic acids of different strengths. Dissolved organic substances from the E horizon were partly sorbed in the BF horizon at the rate of about 65 mg С/100 g, or 22% of Corg in the BF horizon. The sorption resulted in the removal of mainly hydrophobic medium- and high-molecular-weight (>7000 Da) organic substances from the solution. As judged from the coefficients of extinction in the UV area, the sorbed substances were characterized by a higher aromaticity in comparison with the substances remaining in the solution. The sorption of organic substances in the BF horizon was accompanied by the rise in the pH of the solution, which corresponded to binding of organic anions on the surface of iron hydroxides according to the mechanism of ligand exchange with OH ions.
Similar content being viewed by others
References
I. V. Blagodatskikh, Liquid Chromatography of Polymers (Moscow, 2010), p.150.
E. I. Karavanova, “Dissolved organic matter: Fractional composition and sorbability by the soil solid phase (review of literature),” Eurasian Soil Sci. 46, 833–844 (2013). doi 10.1134/S1064229313080048
E. I. Karavanova and L. A. Belyanova, “Composition of soil solutions from the main soil types of the Central State Natural Biosphere Reserve,” Moscow Univ. Soil Sci. Bull. 62, 75–82 (2007).
E. I. Karavanova and M. S. Malinina, “Spatial and temporal variation in the elemental composition of soil solution from gleyic peaty-podzolic soils,” Eurasian Soil Sci. 40, 830–838 (2007).
L. L. Shishov, V. D. Tonkonogov, I. I. Lebedeva, and M. I. Gerasimova, Classification and Diagnostic System of Russian Soils (Oikumena, Smolensk, 2004) [in Russian].
V. N. Kovganko, Methods of Physicochemical Analysis: Laboratory Practicum (Belarusian State Technical Univ., Minsk, 2010) [in Russian].
W. Leithe, Die Analyse der Organischen Verunreinigungen in Trink–, Brauch–und Abwässern (Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1972; Khimiya, Moscow, 1975).
World Reference Base for Soil Resources as the Basis for International Classification and Correlation of Soils, Ed. by V. O. Targulian and M. I. Gerasimova (KMK, Moscow, 2007) [in Russian].
D. S. Orlov, Soil Chemistry (Moscow State Univ., Moscow, 1992), pp. 186–207.
Soil Science, Part 1: Soil and Pedogenesis, Ed. by V. A. Kovda, (Vysshaya Shkola, Moscow, 1988) [in Russian].
Regulatory Role of Soil in Functioning of Taiga Ecosystems, Ed. by G. V. Dobrovol’skii (Nauka, Moscow, 2002) [in Russian].
T. A. Sokolova, T. Ya. Dronova, and I. I. Tolpeshta, Clay Minerals in Soils (Grif i K, Tula, 2005) [in Russian].
T. A. Sokolova, T. Ya. Dronova, I. I. Tolpeshta, and S. E. Ivanova, Interaction of Forest Loamy Podzolic Soils with Model Acid Rains and the Acid-Base Buffer Capacity of Podzolic Soils (Moscow State Univ., Moscow, 2001) [in Russian].
T. A. Sokolova and S. Ya. Trofimov, Sorption Properties of Soils. Adsorption. Cation Exchange (Universitetskaya Kniga, Moscow, 2009), pp. 27–53.
A. D. Shapiro and E. I. Karavanova, “Fractionation of dissolved organic matter on the XAD resin,” Moscow Univ. Soil Sci. Bull. 69, 139–145 (2014).
P. Barré, O. Fernandez-Ugalde, I. Virto, B. Velde, and C. Chenu, “Impact of phyllosilicate mineralogy on organic carbon stabilization in soils: incomplete knowledge and exciting prospects,” Geoderma 235–236, 382–395 (2014).
F.-H. Chi and G. L. Amy, “Kinetic study on the sorption of dissolved natural organic matter onto different aquifer materials: the effects of hydrophobicity and functional groups,” J. Colloid Interface Sci. 274, 380–391 (2004).
J. Chorover and M. K. Amistadi, “Reaction of forest floor organic matter at goethite, birnessite and smectite surfaces,” Geochim. Cosmochim. Acta 65 (1), 95–109 (2001).
W. S. Currie, J. D. Aber, W. H. McDowell, R. D. Boone, and A. H. Magill, “Vertical transport of dissolved organic C and N under long-term N amendments in pine and hardwood forests,” Biogeochemistry 35, 471–505 (1996).
S. K. Deb and M. K. Shukla, “A review of dissolved organic matter transport processes affecting soil and environmental quality,” J. Environ. Anal. Toxicol. 1 (2), 106–117 (2011).
I. Drápelová and J. Kulhavý, “Dissolved organic carbon fluxes in spruce stand through fall and soil water in the Moravian-Silesian Beskids,” Beskydy 2 (1), 9–20 (2009).
R. C. Evanko and A. D. Dzombak, “Influence of structural features on sorption of NOM-analogue organic acids to goethite,” Environ. Sci. Technol. 32, 2846–2855 (1998).
C. J. D. Fraser, MSc Thesis (McGill Univ., Montréal, 1999).
G. Guggenberger and K. Kaiser, “Dissolved organic matter in soil: challenging the paradigm of sorptive preservation,” Geoderma 113, 293–310 (2003).
J. Jaffrain and F. Gurard, “Assessing the quality of dissolved organic matter in forest soils using ultraviolet absorption spectrophotometry,” Soil Sci. Soc. Am. J. 71 (6), 134–139 (2007).
L. K. Johnsen, MSc Thesis (Norwegian University of Life Sciences, Ås, 2011).
K. Kaiser, G. Guggenberger, and W. Zech, “Sorption of DOM and DOM fractions to forest soils,” Geoderma 74, 281–303 (1996).
K. Kaiser and W. Zech, “Dissolved organic matter sorption by mineral constitutes of subsoil clay fraction,” J. Plant Nutr. Soil Sci. 163, 531–535 (2000).
K. Kaiser and W. Zech, “Rates of dissolved organic matter release and sorption in forest soils,” Soil Sci. 163 (9), 714–725 (1998).
K. Kalbitz and K. Kaiser, “Contribution of dissolved organic matter to carbon storage in forest mineral soils,” J. Plant Nutr. Soil Sci. 171, 52–60 (2008).
K. Kalbitz, J. Schmerwitz, D. Schwesig, and E. Matzner, “Biodegradation of soil derived dissolved organic matter as related to its properties,” Geoderma 113 (3–4), 273–291 (2003).
K. Kalbitz, S. Solinger, J.-H. Park, B. Michalzik, and E. Matzner, “Controls on dynamics of dissolved organic matter in soils: a review,” Soil Sci. 165, 277–304 (2000).
M. Kawahigashi, K. Kaiser, A. Rodionov, and G. Guggenberger, “Sorption of dissolved organic carbon by mineral soils of the Siberian forest tundra,” Global Change Biol. 12, 1868–1877 (2006).
R. G. Keil and L. M. Mayer, “Mineral matrices and organic matter,” in Treatise on Geochemistry, Ed. by H. D. Holland and K. K. Turekian (Elsevier, Oxford, 2014), Vol. 12, pp. 337–359.
J. A. Leenheer, “Comprehensive approach to preparative isolation and fractionation of dissolved organic carbon from natural waters and wastewaters,” Environ. Sci. Technol. 15 (5), 578–587 (1981).
M. Meier, K. Namjesnik-Dejanovic, P. A. Maurice, Y.-P. Chin, and G. R. Aiken, “Fractionation of aquatic natural matter upon sorption to goethite and kaolinite,” Chem. Geol. 157, 275–284 (1999).
B. Michalzik, E. Tipping, J. Mulder, J. F. Gallardo Lancho, E. Matzner, C. Bryant, N. Clarke, S. Lofts, and M. A. Vicente Esteban, “Modeling the production and transport of dissolved organic carbon in forest soils,” Biogeochemistry 66, 241–264 (2003).
K. Nambu and K. Yonebayashi, “Acidic properties of dissolved organic matter leached from organic layers in temperate forests,” Soil Sci. Plant Nutr. 45 (1), 65–77 (1999).
J. C. Neff and G. P. Asner, “Dissolved organic carbon in terrestrial ecosystems: synthesis and a model,” Ecosystems 4 (1), 29–48 (2001).
S. C. Nodvin, C. T. Driscoll, and G. E. Likens, “Simple partitioning of anions and dissolved organic carbon in a forest soil,” Soil Sci. 142 (1), 27–35 (1986).
A. Oren and B. Chefetz, “Sorptive and desorptive fractionation of dissolved organic matter by mineral soil matrices,” J. Environ. Qual. 41, 526–533 (2012).
R. G. Quails and L. H. Bruce, “Geochemistry of dissolved organic nutrients in water percolating through a forest ecosystem,” Soil Sci. Soc. Am. J. 55 (4), 1112–1123 (1991).
A. R. Saidy, R. J. Smernik, J. A. Baldock, K. Kaiser, and J. Sanderman, “The sorption of organic carbon onto differing clay minerals in the presence and absence of hydrous iron oxide,” Geoderma 209–210, 15–21 (2013).
D. A. N. Ussiri and C. E. Johnson, “Sorption of organic carbon fractions by spodosol mineral horizons,” Soil Sci. Soc. Am. J. 44, 253–262 (2004).
G. F. Vance and M. B. David, “Chemical characteristics and acidity of soluble organic substances from a northern hardwood forest floor, Central Maine, USA,” Geochim. Cosmochim. Acta 55 (12), 3611–3625 (1991).
J. Vandenbruwane, PhD Thesis (Gent Univ., Gent, 2008).
J. Vandenbruwane, S. D. Neve, R. G. Qualls, S. Sleutel, and G. Hofman, “Comparison of different isotherm models for dissolved organic carbon (DOC) and nitrogen (DON) sorption to mineral soil,” Geoderma 139, 144–153 (2007).
A. Zsolnay, “Dissolved organic matter: artifacts, definitions, and functions,” Geoderma 113 (3–4), 187–209 (2003).
A. Zsolnay, E. Baigar, and M. Jimenez, “Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying,” Chemosphere 38 (1), 45–50 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © D.F. Zolovkina, E.I. Karavanova, A.A. Stepanov, 2018, published in Pochvovedenie, 2018, No. 10, pp. 1215–1225.
Rights and permissions
About this article
Cite this article
Zolovkina, D.F., Karavanova, E.I. & Stepanov, A.A. Sorption of Water-Soluble Organic Substances by Mineral Horizons of Podzol. Eurasian Soil Sc. 51, 1154–1163 (2018). https://doi.org/10.1134/S1064229318100162
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229318100162