Abstract
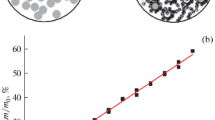
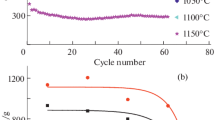
Disperse composite materials based on silicon monoxide and carbon (SiO/C) have been obtained by thermal treatment of a powder mixture consisting of 40 wt % SiO and 60 wt % CF0.8. Annealing has been performed in the argon atmosphere at temperatures 1000–1250°C. It has been established using electron microscopy and Raman scattering that, at T ≥ 1100°C, silicon carbide is formed in the solid-phase product, including in the form of nanowhiskers of cubic modification. Based on the data on the decrease of the reaction mixture weight, the composition of the formed products is calculated as a function of the annealing temperature. The anodes prepared from the composites obtained at a temperature above 1100°C demonstrate a sharp decrease in the capacitance and in the Coulomb efficiency. It is shown that the observed changes are determined by an increase in the oxygen concentration in the matrix surrounding silicon precipitates, which have been formed as a result of SiO disproportionation, rather than by the formation of SiC. It is established that an optimal annealing temperature provides the highest values of the electrode capacitance, the Coulomb efficiency of the first cycle, and the ability to operate at high current densities is T = 1050°C.















Similar content being viewed by others
REFERENCES
K. Pan, F. Zou, M. Canova, Y. Zhu, and J.-H. Kim, J. Power Sources 413, 20 (2019). https://doi.org/10.1016/j.jpowsour.2018.12.010
J. Park, S. S. Park, and Y. S. Won, Electrochim. Acta 107, 467 (2013). https://doi.org/10.1016/j.electacta.2013.06.059
M. Yamada, A. Ueda, K. Matsumoto, and T. Ohzuku, J. Electrochem. Soc. 158 (4), A417 (2011). https://doi.org/10.1149/1.3551539
T. Tan, P.-K. Lee, and D. Y. W. Yu, J. Electrochem. Soc. 166 (3), A5210 (2019). https://doi.org/10.1149/2.0321903jes
E. V. Astrova, V. P. Ulin, A. V. Parfeneva, and V. B. Voronkov, Tech. Phys. Lett. 45 (7), 664 (2019). https://doi.org/10.1134/S1063785019070022
E. V. Astrova, V. P. Ulin, A. V. Parfeneva, A. M. Rumyantsev, V. B. Voronkov, A. V. Nashchekin, V. N. Nevedomskiy, Y. M. Koshtyal, and M. V. Tomkovich, J. Alloy Compd. 826, 154242 (2020). https://doi.org/10.1016/j.jallcom.2020.154242
E. V. Astrova, A. V. Parfeneva, A. M. Rumyantsev, V. P. Ulin, M. V. Baidakova, V. N. Nevedomskii, and A. V. Nashchekin, Tech. Phys. Lett. 46 (2), 114 (2020). https://doi.org/10.1134/S1063785020020042
E. V. Astrova, V. P. Ulin, A. V. Parfeneva, A. V. Nashchekin, V. N. Nevedomskiy, and M. V. Baidakova, Semiconductors 54 (8), 900 (2020). https://doi.org/10.1134/S1063782620080059
D. A. Lozhkina, E. V. Astrova, R. V. Sokolov, D. A. Kirilenko, A. A. Levin, A. V. Parfeneva, and V. P. Ulin, Semiconductors 55 (4), 373 (2021). https://doi.org/10.1134/S1063782621040096
Ch.-M. Park, W. Choi, Y. Hwa, J.-H. Kim, G. Jeong, and H.-J. Sohn, J. Mater. Chem. 20, 4854 (2010). https://doi.org/10.1039/B923926J
D. Sri Maha Vishnu, J. Sure, H.-K. Kim, R. Vasant Kumar, and C. Schwandt, Energy Storage Mater. 26, 234 (2020). https://doi.org/10.1016/j.ensm.2019.12.041
Y. Hu, X. Liu, X. Zhang, N. Wan, D. Pan, X. Li, Y. Bai, and W. Zhang, Electrochim. Acta 190, 33 (2016). https://doi.org/10.1016/j.electacta.2015.12.211
M. Wojdyr, J. Appl. Crystallogr. 43 (5), 1126 (2010). https://doi.org/10.1107/S0021889810030499
R. Dhiman, E. Johnson, and P. Morgen, Ceram. Int. 37 (8), 3759 (2011). https://doi.org/10.1016/j.ceramint.2011.06.001
M. Bechelany, A. Brioude, D. Cornu, G. Ferro, and P. Miele, Adv. Funct. Mater. 17, 939 (2007). https://doi.org/10.1002/adfm.200600816
S.-L. Zhang, B.-F. Zhu, F. Huang, Y. Yan, E.-Y. Shang, S. Fan, and W. Han, Solid State Commun. 111, 647 (1999). https://doi.org/10.1016/S0038-1098(99)00262-8
A. Merlen, J. G. Buijnsters, and C. Pardanaud, Coatings 7 (10), 153 (2017). https://doi.org/10.3390/coatings7100153
A. Sadezky, H. Muckenhuber, H. Grothe, R. Niessner, and U. Pöschl, Carbon 43 (8), 1731 (2005). https://doi.org/10.1016/j.carbon.2005.02.018
A. Ya. Vinogradov, S. A. Grudinkin, N. A. Besedina, S. V. Koniakhin, M. K. Rabchinskii, E. D. Eidelman, and V. G. Golubev, Semiconductors 52 (7), 914 (2018). https://doi.org/10.1134/S1063782618070266
A. C. Ferrari and J. Robertson, Phys. Rev. B 61 (20), 14095 (2000). https://doi.org/10.1103/PhysRevB.61.14095
F. Tuinstra and J. L. Koening, J. Chem. Phys. 53, 1126 (1970). https://doi.org/10.1063/1.1674108
M. S. Dresselhaus, M. A. Pimenta, P. C. Eklund, and G. Dresselhaus, in Raman Scattering in Materials Science, Ed. by W. H. Weber and R. Merlin (Springer, Berlin, 2000), Vol. 42, p. 314.
L. G. Cancado, K. Takai, T. Enoki, M. Endo, Y. A. Kim, H. Mizusaki, N. L. Speziali, A. Jorio, and M. A. Pimenta, Carbon 46, 272 (2008). https://doi.org/10.1016/j.carbon.2007.11.015
T. P. Nguyen and S. Lefrant, Solid State Commun. 57 (4), 235 (1986). https://doi.org/10.1016/0038-1098(86)90146-8
ACKNOWLEDGMENTS
Electron microscopic investigations were performed on the equipment of the Joint Research Center “Materials Science and Diagnostics in Advanced Technologies” of the Ioffe Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by N. Wadhwa
Rights and permissions
About this article
Cite this article
Lozhkina, D.A., Astrova, E.V., Likhachev, A.I. et al. Silicon Monoxide Carbonized by Fluorocarbon As a Composite Material for Anodes of Lithium-Ion Batteries. Tech. Phys. 66, 1228–1240 (2021). https://doi.org/10.1134/S1063784221090103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063784221090103