Abstract
It has been shown that three singular points related to an amorphous state can be distinguished in the dependence of the first coordination number kn on packing coefficient kp in the structure of a unicomponent material. The equation of state and properties of iron for both these three amorphous structures and the crystal state are calculated on the basis of the Mie–Lennard-Jones pair interatomic potential. It is shown that the chemical potential becomes minimal at kp = 0.45556 and kn = 6.2793; that is, this packing can be assigned to a thermodynamically stable amorphous structure corresponding to a liquid phase. A point that is energetically equivalent to this phase and has the same value of kn but another value of kp (0.6237) is a thermodynamically unstable amorphous structure corresponding to a solid phase. It was shown that the specific surface energy of an amorphous solid metal is higher than that of the amorphous liquid phase but is lower than the specific surface energy of a crystalline metal. This should lead to the fact that the surface of a crystalline metal will tend to amorphization.





Similar content being viewed by others
Notes
Hereafter, approximation by polynomials of different degrees is done by the least-squares method embedded in the Origin graphic plotter.
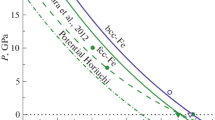
The value T = 10 K was taken to show how the S-shaped loop varies on the fH(kp) dependence with temperature. At T = 10 K, the liquid phase cannot be in equilibrium with the solid (amorphous or crystalline) one.
REFERENCES
D. K. Belashchenko, Computer Simulation of Liquid and Amorphous Substances (MISIS, Moscow, 2005) [in Russian].
R. N. Singh and I. Ali, Int. J. Appl. Phys. Math. 3 (4), 275 (2013). https://doi.org/10.7763/IJAPM.2013.V3.220
G. Melnikov, S. Emelyanov, N. Ignatenko, and O. Manzhos, Key Eng. Mater. 781, 137 (2018). https://doi.org/10.4028/www.scientific.net/KEM.781.137.
A. Baule, F. Morone, H. J. Herrmann, and H. A. Makse, Rev. Mod. Phys. 90 (1), 015006 (2018). https://doi.org/10.1103/RevModPhys.90.015006
M. N. Magomedov, J. Struct. Chem. 49 (1), 156 (2008). https://doi.org/10.1007/s10947-008-0021-8
V. I. Kalikmanov, J. Chem. Phys. 124 (12), 124505 (2006). https://doi.org/10.1063/1.2178812
C. Song, P. Wang, and H. A. Makse, Nature 453 (7195), 629 (2008). https://doi.org/10.1038/nature06981
M. Hanifpour, N. Francois, V. Robins, A. Kingston, S. V. Allaei, and M. Saadatfar, Phys. Rev. E 91 (6), 062202 (2015). https://doi.org/10.1103/PhysRevE.91.062202
M. N. Magomedov, Tech. Phys. Lett. 45 (10), 1042 (2019). https://doi.org/10.1134/S1063785019100249
W. G. Hoover and F. H. Ree, J. Chem. Phys. 49 (8), 3609 (1968). https://doi.org/10.1063/1.1670641
M. N. Magomedov, Study of Interatomic Interaction, Vacancy Formation and Self-Diffusion in Crystals (Fizmatlit, Moscow, 2010) [in Russian].
M. N. Magomedov, Tech. Phys. 58 (9), 1297 (2013). https://doi.org/10.1134/S106378421309020X
L. A. Girifalco, Statistical Physics of Materials (Wiley, New York, 1973).
M. N. Magomedov, Tech. Phys. 60 (11), 1619 (2015). https://doi.org/10.1134/S1063784215110195
M. N. Magomedov, Crystallogr. Rep. 62 (3), 480 (2017). https://doi.org/10.1134/S1063774517030142
E. F. Pichugin, Izv. Vyssh. Uchebn. Zaved., Fiz., No. 6, 77 (1962).
M. N. Magomedov, Tech. Phys. 62 (4), 569 (2017). https://doi.org/10.1134/S1063784217040156
X. Huang, F. Li, Q. Zhou, Y. Meng, K. D. Litasov, X. Wang, B. Liu, and T. Cui, Sci. Rep. 6 (1–3), 19923 (2017). https://doi.org/10.1038/srep19923
S. S. Batsanov and A. S. Batsanov, Introduction to Structural Chemistry (Springer, Heidelberg, 2012). https://doi.org/10.1007/978-94-007-4771-5
Y. R. Luo, “Bond Dissociation Energies,” in CRC Handbook of Chemistry and Physics, Ed. by D. R. Lide, 90th ed. (CRC Press, Boca Raton, Florida, 2009). https://notendur.hi.is/agust/rannsoknir/papers/2010-91-CRC-BDEs-Tables.pdf.
V. E. Zinov’ev, The Thermophysical Proper ties of Metals at High Temperatures (Metallurgiya, Moscow, 1989) [in Russian].
D. R. Wilburn and W. A. Bassett, Am. Mineral. 63 (5–6), 591 (1978). https://pubs.geoscienceworld.org/msa/ammin/article-abstract/63/5-6/591/40926.
Handbook of Physical Quantities, Ed. by I. S. Grigoriev and E.Z. Meilikhov (CRC Press, Boca Raton, Florida, 1996).
S. I. Novikova, Thermal Expansion of Solids (Nauka, Moscow, 1974) [in Russian].
V. K. Kumikov and Kh. B. Khokonov, J. Appl. Phys. 54 (3), 1346 (1983). https://doi.org/10.1063/1.332209
V. K. Kumikov, Mater. Sci. Eng. 60 (3), L23 (1983). https://doi.org/10.1016/0025-5416(83)90016-2
Y. Li and G. A. Somorjai, J. Phys. Chem. C 111 (27), 9631 (2007). https://doi.org/10.1021/jp071102f
V. P. Bokarev, E. S. Gornev, and P. A. Todua, Kristallografiya 58 (1), 155 (2013). https://doi.org/10.7868/S0023476113010062
P. N. Pusey and W. van Megen, Nature 320 (6060), 340 (1986). https://doi.org/10.1038/320340a0
M. Campo and T. Speck, J. Chem. Phys. 152 (1), 014501 (2020). https://doi.org/10.1063/1.5134842
ACKNOWLEDGMENTS
The author thanks S.P. Kramynin, N.Sh. Gazanova, Z.M. Surkhaeva, and M.M. Gadzhieva for fruitful discussions and assistance.
Funding
This study was supported by the Russia Foundation for Basic Research (grant no. 18-29-11013_mk) and program no. 6 of the Presidium of the Russian Academy of Sciences (grant no. 2-13).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest.
Additional information
Translated by V. Isaakyan
Rights and permissions
About this article
Cite this article
Magomedov, M.N. An Equation of the State and Surface Properties of Amorphous Iron. Tech. Phys. 65, 1659–1665 (2020). https://doi.org/10.1134/S1063784220100138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063784220100138