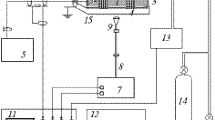
Abstract
The conditions for the formation of a plasma discharge in an oxygen-containing ion solution are analyzed. Starting regimes of plasma excitation, which depend on physicochemical properties of the electrolyte, electrode, released gas, and current density, are substantiated theoretically. The description of the process is based on electrode screening by gas bubbles. The critical values of the geometrical and true current density (9.7 and 143.5 × 104 A/m2), the extent of screening of the electrode by hydrogen bubbles (93.2%), the rate of emergence of bubbles (1.6 × 10-6 s-1), and other parameters preceding the evolution of the plasma are determined for the 1% aqueous solution of sulfuric acid. The results of calculations are confirmed in experiments. A method is proposed for determining the limiting wetting angle Θ of the electrode surface from the kinetics of emergence of bubbles. For the electrolyte and copper electrode used in experiments (T = 343 K, j = 4.18 A/m2), this angle was found to be 5.0° ± 0.1° and 175.0° ± 0.1° for hydrogen bubbles.
Similar content being viewed by others
References
E. D. Mishina, K. A. Vorotilov, V. A. Vasil’ev, et al., Zh. Eksp. Teor. Fiz. 122, 582 (2002) [JETP 93, 502 (2002)].
J. Choi, K. Nielsch, M. Reiche, et al., J. Vac. Sci. Technol. B 21, 763 (2003).
S. Ono, M. Saito, M. Ishiguro, et al., J. Electrochem. Soc. 151, 473 (2004).
V. S. Teslenko, A. P. Drozhzhin, and G. N. Sankin, Pis’ma Zh. Tekh. Fiz. 32(4), 24 (2006) [Tech. Phys. Lett. 32, 149 (2006)].
E. E. Aver’yanov, Handbook of Anodic Treatment, Ed. by E.V. Medvedev (Mashinostroenie, Moscow, 1988) [in Russian].
Yu. N. Tyurin and A. D. Pogrebnyak, Zh. Tekh. Fiz. 72(11), 119 (2002) [Tech. Phys. 47, 1463 (2002)].
A. D. Pogrebnyak, O. P. Kul’ment’eva, A. P. Kobzev, et al., Pis’ma Zh. Tekh. Fiz. 29(8), 8 (2003) [Tech. Phys. Lett. 29, 312 (2003)].
K. N. Eretnev amd S. V. Lebedev, Processes of Heating and Cleaning of Metal Surfaces in Electrolytes and Their Applications (Lipetsk, 1977) [in Russian].
Handbook of Chemist, Ed. by B. P. Nikol’skii (Khimiya, Moscow-Leningrad, 1966), Vol. 1.
N. P. Bogoroditskii and V. V. Pasynkov, Materials in Radioelectronics (Gosenergoizdat, Moscow-Lenin-grad, 1961) [in Russian].
A. Lisovskii and S. D. Yakovin, Pis’ma Zh. Eksp. Teor. Fiz. 72(2), 49 (2000) [JETP Lett. 72, 34 (2000)].
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.M. Orlov, I.O. Yavtushenko, A.V. Zhuravleva, 2010, published in Zhurnal Tekhnicheskoǐ Fiziki, 2010, Vol. 80, No. 2, pp. 60–65.
Rights and permissions
About this article
Cite this article
Orlov, A.M., Yavtushenko, I.O. & Zhuravleva, A.V. Starting regimes of plasma excitation in conducting aqueous solutions. Tech. Phys. 55, 219–224 (2010). https://doi.org/10.1134/S106378421002009X
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106378421002009X