Abstract
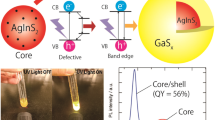
The structural and optical properties of colloidal Ag2S quantum dots in different environments are studied. With the help of transmission electron microscopy, X-ray diffraction analysis, and X-ray Energy-Dispersive analysis, the formation of Ag2S quantum dots with average size of 2–3 nm with monoclinic crystal lattice, and Ag2/SiO2 core–shell systems based on them, has been enstablished. A change in the luminescence quantum yield of the quantum dots in the case of a change of the surface environment is shown. Decoration of TiO2 nanoparticles with a size of 10–15 nm with Ag2S quantum dots is performed and the effect of the structure of the interfaces of the quantum dots and their environment (2-mercaptopropionic acid, water, ethylene glycol, SiO2 dielectric shells with thicknesses of 0.6 and 2.0 nm) on the formation of TiO2–Ag2S heterosystem is analyzed. The signs of charge phototransfer upon adsorption onto the surface of TiO2 nanoparticles are found for the Ag2S quantum dots passivated with 2-mercaptopropionic acid. The signs of appearance of reactive oxygen species as a result of charge phototransfer in a TiO2–Ag2S heterosystem are found based on the observation of photobleaching of methylene blue upon excitation of the system from the region outside of TiO2 fundamental absorption.






Similar content being viewed by others
REFERENCES
H. L. Chou, B.-J. Hwang, and C.-L. Sun, in New Future Developments in Catalysis (Elsevier, Amsterdam, 2013), p. 217. https://doi.org/10.1016/B978-0-444-53880-2.00014-4
J. J. Ng, K. H. Leong, L. C. Sim, W.-D. Oh, C. Dai, and P. Saravanan, in Nanomaterials for Air Remediation (Elsevier, Amsterdam, 2020), p. 193. https://doi.org/10.1016/B978-0-12-818821-7.00010-5
M. Sakar, R. M. Prakash, K. Shinde, and G. R. Balakrishna, Int. J. Hydrogen Energy 45, 7691 (2020). https://doi.org/10.1016/j.ijhydene.2019.04.222
A. Kubackaa, U. Caudillo-Flores, I. Barba-Nieto, and M. Fernández-García, Appl. Catal. A: Gen. 610, 117966 (2021). https://doi.org/10.1016/j.apcata.2020.117966
S. Shen, C. Kronawitter, and G. Kiriakidis, J. Materiomics 3, 1 (2017).https://doi.org/10.1016/j.jmat.2016.12.004
M. Pawar, S. T. Sendoğdular, and P. Gouma, J. Nanomater. 2018, 5953609 (2018). https://doi.org/10.1016/j.jmat.2016.12.004
A. L. Linsebigler, G. Lu, and J. T. Yates, Jr., Chem. Rev. 95, 735 (1995). https://doi.org/10.1021/cr00035a013
K. Nakata and A. Fujishima, J. Photochem. Photobiol. C 13, 169 (2012). https://doi.org/10.1016/j.jphotochemrev.2012.06.001
J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo, and D. W. Bahnemann, Chem. Rev. 114, 9919 (2014). https://doi.org/10.1021/cr5001892
Z. Bao, S. Wang, X. Yu, Y. Gao, and Z. Wen, Water Air Soil Pollut. 230, 169 (2019). https://doi.org/10.1007/s11270-019-4219-5
O. R. Fonseca-Cervantes, A. Perez-Larios, V. H. Ro-mero Arellano, B. Sulbaran-Rangel, and C. A. Guzman Gonzalez, Processes 8, 1032 (2020). https://doi.org/10.3390/pr8091032
M. Janczarek and E. Kowalska, Catalysts 7, 317 (2017). https://doi.org/10.3390/catal7110317
S. B. Rawal, S. Bera, D. Lee, D.-J. Jang, and W. In Lee, Catal. Sci. Technol. 3, 1822 (2013). https://doi.org/10.1039/C3CY00004D
C. del Cacho, O. Geiss, P. Leva, S. Tirendi, and J. Barrero-Moreno, in Nanotechnology in Eco-Efficient Construction (Woodhead, 2013), p. 343. https://doi.org/10.1533/9780857098832.3.343
I. Zumeta-Dube, V.-F. Ruiz-Ruiz, D. Diaz, S. Rodil-Posadas, and A. Zeinert, Phys. Chem. C 118, 11495 (2014). https://doi.org/10.1021/jp411516a
A. Badawi, Phys. E (Amsterdam, Neth.) 109, 107 (2019). https://doi.org/10.1016/j.physe.2019.01.018
R. Gui, H. Jin, Z. Wang, and L. Tan, Coord. Chem. Rev. 296, 91 (2015). https://doi.org/10.1016/j.ccr.2015.03.023
R. Gui, A. Wan, X. Liu, W. Yuan, and H. Jin, Nanoscale 6, 5467 (2014). https://doi.org/10.1039/C4NR00282B
R. Gui, J. Sun, D. Liu, Y. Wang, and H. Jin, Dalton Trans. 43, 16690 (2014). https://doi.org/10.1039/C4DT00699B
R. Tang, J. Xue, B. Xu, D. Shen, G. P. Sudlow, and S. Achilefu, ACS Nano 9, 220 (2015). https://doi.org/10.1021/nn5071183
Y. Xie, S. H. Yoo, C. Chen, and S. Oh, Mater. Sci. Eng. B 177, 106 (2012). https://doi.org/10.1016/j.mseb.2011.09.021
B. Liu, D. Wang, Y. Zhang, H. Fan, Y. Lin, T. Jiang, and T. Xie, Dalton Trans. 42, 2232 (2014). https://doi.org/10.1039/C2DT32031B
K. Nagasuna, T. Akita, M. Fujishima, and H. Tada, Langmuir 27, 7294 (2011). https://doi.org/10.1021/la200587s
M. Smirnov and O. Ovchinnikov, J. Lumin. 227, 117526 (2020). https://doi.org/10.1016/j.jlumin.2020.117526
O. V. Ovchinnikov, I. G. Grevtseva, M. S. Smirnov, T. S. Kondratenko, A. S. Perepelitsa, S. V. Aslanov, V. U. Khokhlov, E. P. Tatyanina, and A. S. Matsukovich, Opt. Quantum Electron. 52, 198 (2020). https://doi.org/10.1007/s11082-020-02314-8
O. V. Ovchinnikov, M. S. Smirnov, B. I. Shapiro, T. S. Shatskikh, A. S. Perepelitsa, and N. V. Korolev, Semiconductors 49, 373 (2015). https://doi.org/10.1134/S1063782615030173
C. M. Wilke, C. Petersen, M. A. Alsina, J.-F. Gaillard, and K. A. Gray, Environ. Sci.: Nano 6, 115 (2019). https://doi.org/10.1039/C8EN01159A
X. Liu, L. Zhu, X. Wang, and X. Meng, Env. Sci. Pollut. Res. 27, 13590 (2020). https://doi.org/10.1007/s11356-020-07960-9
T. S. Kondratenko, M. S. Smirnov, O. V. Ovchinnikov, A. I. Zvyagin, T. A. Chevychelova, and I. V. Taydakov, Bull. Lebedev Phys. Inst. 46, 210 (2019). https://doi.org/10.3103/S106833561906006X
O. V. Ovchinnikov, S. V. Aslanov, M. S. Smirnov, A. S. Perepelitsa, T. S. Kondratenko, A. S. Selyukov, and I. G. Grevtseva, Opt. Mater. Express 11, 89 (2021). https://doi.org/10.1364/OME.411432
O. V. Ovchinnikov, S. V. Aslanov, M. S. Smirnov, I. G. Grevtseva, and A. S. Perepelitsa, RSC Adv. 9, 37312 (2019).https://doi.org/10.1039/C9RA07047H
J. R. Lakowicz, Principles of Fluorescence Spectroscopy (Springer, New York, 2006). https://doi.org/10.1007/978-0-387-46312-4
S. Reindl, A. Penzkofer, S.-H. Gong, M. Landthaler, R. M. Szeimies, C. Abels, and W. Baumler, J. Photochem. Photobiol. A 105, 65 (1997). https://doi.org/10.1016/S1010-6030(96)04584-4
A. J. Frueh, Z. Kristallogr. 110, 136 (1958).
H. F. Poulsen, J. Neuefeind, H.-B. Neumann, and J. R. Schneider, J. Non-Cryst. Solids 188, 63 (1995). https://doi.org/10.1016/0022-3093(95)00095-X
S. Music, N. Filipovic-Vincekovic, and L. Sekovanic, Braz. J. Chem. Eng. 28, 89 (2011). https://doi.org/10.1590/S0104-66322011000100011
H. Ijadpanah-Saravy, M. Safari, A. Khodadadi-Darban, and A. Rezaei, Anal. Lett. 47, 1772 (2014). https://doi.org/10.1080/00032719.2014.880170
S. Lin, Y. Feng, X. Wen, P. Zhang, S. Woo, S. Shrestha, G. Conibeer, and S. Huang, J. Phys. Chem. 119, 867 (2015). https://doi.org/10.1021/jp511054g
Y. Kayanuma, Phys. Rev. B 38, 9797 (1988). https://doi.org/10.1103/PhysRevB.38.9797
A. B. Murphy, Sol. Energy Mater. Sol. Cells 91, 1326 (2007). https://doi.org/10.1016/j.solmat.2007.05.005
V. M. Ievlev, C. B. Kushchev, A. N. Latyshev, L. Yu. Leonova, O. V. Ovchinnikov, M. S. Smirnov, E. V. Popova, A. V. Kostyuchenko, and S. A. Soldatenko, Semiconductors 48, 848 (2014).
A. S. Perepelitsa, O. V. Ovchinnikov, M. S. Smirnov, T. S. Kondratenko, I. G. Grevtseva, S. V. Aslanov, and V. Y. Khokhlov, J. Lumin. 231, 117805 (2021). https://doi.org/10.1016/j.jlumin.2020.117805
A. Mills and J. Wang, J. Photochem. Photobiol. A 124, 123 (1999). https://doi.org/10.1016/S1010-6030(99)00143-4
S. Otsuka-Yao-Matsuo, T. Omata, S. Ueno, and M. Kita, Mater. Trans. 44, 2124 (2003).
J. Yao and C. Wang, Int. J. Photoenergy 2010, 643182 (2010). https://doi.org/10.1155/2010/643182
ACKNOWLEDGMENTS
The structural studies performed on a THERMO ARL X’TRA diffractometer (ThermoFisher, Switzerland) and a LIBRA 120 transmission electron microscope (CarlZeiss, Germany) were performed using the equipment of the Center for Collective Use of Scientific Equipment of the Voronezh State University.
Funding
This work was financially supported by the Russian Foundation for Basic Research (grant no. 20-32-90167 Aspiranty).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Ovchinnikov, O.V., Smirnov, M.S., Aslanov, S.V. et al. Luminescent Properties of Colloidal Ag2S Quantum Dots for Photocatalytic Applications. Phys. Solid State 64, 71–79 (2022). https://doi.org/10.1134/S1063783422010140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783422010140