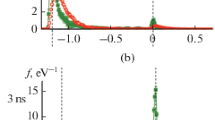
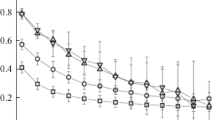
Abstract
The condensation of metal vapor in an inert gas is studied by the molecular dynamics method. Two condensation regimes are investigated: with maintenance of partial pressure of the metal vapor and with a fixed number of metal atoms in the system. The main focus is the study of the cluster energy distribution over the degrees of freedom and mechanisms of the establishment of thermal equilibrium. It is shown that the internal temperature of a cluster considerably exceeds the buffer gas temperature and the thermal balance is established for a time considerably exceeding the nucleation time. It is found that, when the metal vapor concentration exceeds 0.1 of the argon concentration, the growth of clusters with the highest possible internal energy occurs, the condensation rate being determined only by the rate of heat removal from clusters.
Similar content being viewed by others
References
Inorganic Nanoparticles, Ed. by C. Altavilla and E. Ciliberto (CRC Press, New York, 2011).
C. G. Granqvist and R. A. Buhrman, J. Appl. Phys. 47, 2200 (1976).
K. Sattler, J. Muhlbach, and E. Recknagel, Phys. Rev. Lett. 45, 821 (1980).
A. Simchi, R. Ahmadi, S. M. S. Reihani, and A. Mahdavi, Mater. Des. 28, 850 (2007).
I. V. Frishberg, L. I. Kvater, B. P. Kuz’min, and S. V. Gribovskii, The Gas-Phase Method of Obtaining Powders (Nauka, Moscow, 1978) [in Russian].
M. Volmer and A. Weber, Z. Phys. Chem. 119, 227 (1926).
R. Becker and W. Doring, Ann. Phys. (Weinheim) 24, 719 (1935).
Ya. B. Zel’dovich, Zh. Eksp. Teor. Fiz. 12, 525 (1942).
Ya. I. Frenkel’, Zh. Eksp. Teor. Fiz. 9, 952 (1939).
K. Yasuoka and M. Matsumoto, J. Chem. Phys. 109, 8451 (1998).
V. I. Kalikmanov, J. Wolk, and T. Kraska, J. Chem. Phys. 128, 124506 (2008).
B. J. Alder and T. E. Wainwright, J. Chem. Phys. 31, 459 (1960).
A. S. Barnard, Rep. Prog. Phys. 73, 086502 (2010).
F. Baletto and R. Ferrando, Rev. Mod. Phys. 77(1), 371 (2005).
S. L. Gafner, S. V. Kosterin, and Yu. Ya. Gafner, Phys. Solid State 49(8), 1558 (2007).
Y. Wang, S. Teitel, and C. Dellago, J. Chem. Phys. 122, 214722 (2005).
Yu. Ya. Gafner, S. L. Gafner, and I. V. Chepkasov, JETP 111(4), 608 (2010).
S. L. Gafner and Yu. Ya. Gafner, JETP 107(4), 712 (2008).
P. Krasnochtchekov and R. S. Averback, J. Chem. Phys. 122, 044319 (2005).
F. Romer and T. Kraska, J. Chem. Phys. 127, 234509 (2007).
E. Kesala, A. Kuronen, and K. Nordlund, Phys. Rev. B: Condens. Matter 75, 174121 (2007).
J. Westergren, H. Grönbeck, S.-G. Kim, and D. Tománek, J. Chem. Phys. 107, 3071 (1997).
K. Yasuoka and X. C. Zeng, J. Chem. Phys. 126, 124320 (2007).
S. Braun, F. Römer, and T. Kraska, J. Chem. Phys. 131, 064308 (2009).
N. Lümmen and T. Kraska, Comput. Mater. Sci. 35, 210 (2006).
S. Plimpton, J. Comput. Phys. 117, 1 (1995).
S. M. Foiles, M. I. Baskes, and M. S. Daw, Phys. Rev. B: Condens. Matter 33, 7983 (1986).
H. C. Andersen, J. Chem. Phys. 72, 2384 (1972).
S. Nose, J. Chem. Phys. 81, 511 (1984).
I. V. Maltsev, A. A. Mirzoev, D. A. Danilov, and B. Nestler, Modell. Simul. Mater. Sci. Eng. 17, 055006 (2009).
Handbook of Physical Quantities, Ed. by I. S. Grigoriev and E. Z. Meilikhov (Energoatomizdat, Moscow, 1991; CRC Press, Boca Raton, Florida, United States, 1997).
F. H. Stillinger, J. Chem. Phys. 38, 1486 (1963).
M. P. Allen and D. J. Tildesly, Computer Simulation of Liquids (Clarendon, Oxford, 1990).
A. G. Vorontsov, Vestn. Yuzhno-Ural. Gos. Univ., Ser. “Mat. Mekh. Fiz.,” No. 1, 39 (2009).
B. R. Gel’chinskii, A. G. Vorontsov, A. E. Korenchenko, and L. I. Leont’ev, Dokl. Phys. Chem. 436(Part 2), 15 (2011).
A. G. Vorontsov, B. R. Gel’chinskii, and A. E. Korenchenko, Vestn. Yuzhno-Ural. Gos. Univ., Ser. “Mat. Mekh. Fiz.,” No. 4, 61 (2011).
Handbook of the Physicochemical Properties of the Elements, Part 1: Physical Properties, Ed. by G. V. Samsonov (Plenum, New York, 1968; Metallurgiya, Moscow, 1976).
S. M. Thompson, K. E. Gubbins, J. Walton, R. A. R. Chantry, and J. S. Rowlinson, J. Chem. Phys. 81, 530 (1984).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.G. Vorontsov, B.R. Gel’chinskii, A.E. Korenchenko, 2012, published in Zhurnal Eksperimental’noi i Teoreticheskoi Fiziki, 2012, Vol. 142, No. 5, pp. 897–907.
Rights and permissions
About this article
Cite this article
Vorontsov, A.G., Gel’chinskii, B.R. & Korenchenko, A.E. Kinetics and energy states of nanoclusters in the initial stage of homogeneous condensation at high supersaturation degrees. J. Exp. Theor. Phys. 115, 789–797 (2012). https://doi.org/10.1134/S1063776112100160
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063776112100160