Abstract
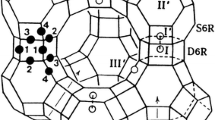
The crystal structures of ion-exchange products of the zeolite-group mineral amicite from Khibiny Alkaline Massif with silver and rubidium salts have been studied. The investigated samples are monoclinic (sp. gr. P21 and I2/a); their crystallochemical formulas (Z = 1) are Ag8(Al8Si8O32) ⋅ 10.6H2O and K0.4Na0.7Rb6.9(Al8Si8O32) ⋅ 4.5H2O; the unit cell parameters are a = 9.272(2) and 10.043(2) Å, b = 9.997(2) and 9.748(2) Å, c = 10.380(3) and 10.265(2) Å, β = 90.821(8)° and 90.09(3)°, V = 962.0(4) and 1005.0(3) Å3, respectively. In the Ag-amicite structure, 22 partially occupied sites of the extra-framework components (Ag and H2O) are localized. In the Rb-amicite structure, most of rubidium atoms are located in two sites with the occupancies of 0.414(6) and 0.389(5). The cross-sectional areas of the zeolite channels are 3.5 × 4.5 and 2.3 × 6.3 Å2 for Ag-amicite and 3.0 × 4.3 and 3.0 × 3.9 Å2 for Rb-amicite.


Similar content being viewed by others
REFERENCES
G. Artioli, Am. Mineral. 77, 189 (1992). https://pubs.geoscienceworld.org/msa/ammin/article-abstract/77/1-2/189/42585/The-crystal-structure-of-garronite?redirectedFromfulltext.
L. B. McCusker, C. Baerlocher, and R. Nawaz, Z. Kristallogr. 171, 281 (1985). https://rruff.info/doclib/zk/vol171/ZK171_281.pdf.
K. Fischer and V. Schramm, Adv. Chem. Ser.: Mol. Sieve Zeolites 101, 250 (1970).
A. Alberti, G. Hentschel, and G. Vezzalini, Neues Jb. Miner. Monatsh. 481 (1979).
A. P. Khomyakov, G. E. Cherepivskaya, T. A. Kurova, et al., Dokl. Akad. Nauk SSSR 263, 978 (1982).
I. V. Pekov and A. S. Podlesnyi, Kukisvumchorr Deposit: Mineralogy of Alkaline Pegmatites and Hydrothermalites. Mineralogical Almanac (Ocean Pictures, Littleton, 2004), Vol. 7.
A. Alberti and G. Vezzalini, Acta Crystallogr. B 35, 2866 (1979). https://doi.org/10.1107/S0567740879010852
T. Bauer and W. Baur, Eur. J. Mineral. 10, 133 (1998). https://doi.org/10.1127/ejm/10/1/0133
C. J. Adams, A. Araya, S. W. Carr, et al., Recent Progress and Discussions Studies in Surface Science and Catalysis, Ed. by H. G. Karge and J. Weitkamp (Elsevier, Amsterdam, 1995), Vol. 98, p. 206.
S. Allen, S. Carr, A. Chapple, et al., Phys. Chem. Chem. Phys. 4, 2409 (2002). https://doi.org/10.1039/b111490p
A. S. Pakhomova, R. M. Danisi, T. Armbruster, et al., Microporous Mesoporous Mater. 182, 207 (2013). https://doi.org/10.1016/j.micromeso.2013.08.036
G. Vezzalini, A. Alberti, A. Sani, and M. Triscari, Microporous Mesoporous Mater. 31, 253 (1999). https://doi.org/10.1016/S1387-1811(99)00076-1
Agilent Technologies, CrysAlisPro Software system, Version 1.171.36.20 (Agilent Technologies UK Ltd., Oxford, UK, 2012).
G. M. Sheldrick, Acta Crystallogr. A 64, 112 (2008). https://doi.org/10.1107/S0108767307043930
G. M. Sheldrick, Acta Crystallogr. C 71, 3 (2015). https://doi.org/10.1107/S2053273314026370
N. V. Chukanov, O. N. Kazheva, N. A. Chervonnaya, et al., Maced. J. Chem. Chem. Eng. 39, 207 (2020). https://doi.org/10.20450/mjcce.2020.1984
Funding
The work was supported by the State assignment (registration no. AAAA-A19-119092390076-7) in the part concerning X-ray diffraction analysis and by the Russian Foundation for Basic Research, project no. 18-29-12007-MK, in the part concerning the determination of the chemical composition determination and ion-exchange experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Bondareva
Rights and permissions
About this article
Cite this article
Kazheva, O.N., Chukanov, N.V., Chervonnaya, N.A. et al. Crystal Structures of Rb- and Ag-Substituted Forms of Natural Zeolite Amicite. Crystallogr. Rep. 66, 86–94 (2021). https://doi.org/10.1134/S1063774521010089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774521010089