Abstract
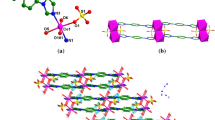
A coordination polymer, {[Co(bib)3](BF4)2}n, was prepared for the first time at room temperature via the reaction between cobalt(II) chloride and flexible linker ligand, 1,4-bis(imidazolyl)butane (bib), in the presence of sodium tetrafluoroborate using a three layers method. The compound was characterized by elemental analysis, infrared spectroscopy, and single-crystal X-ray diffraction. It crystallizes in the triclinic sp. gr. \(P\bar {1}\) with the unit cell parameters a = 9.8124(2), b = 22.1804(4), c = 26.9233(5) Å and α = 71.910(2)°, β = 89.418(2)°, γ = 86.992(2)°. In the polymeric structure of the compound, the cobalt(II) ions have the CoN6 octahedral geometry and six bib-ligands are coordinated to one metal ion to form an open 3D 2-fold interpenetrated framework (α-polonium-type net, pcu).




Similar content being viewed by others
REFERENCES
H. L. Yu, Q. Zhang, Y. Wang, and J. Duan, Dalton Trans. 47, 4424 (2018).
J. Sim, H. Yim, N. Ko, et al., Dalton Trans. 43, 18017 (2014).
S. Wang, J. Liu, H. Zhao, et al., Inorg. Chem. 57, 541 (2017).
B. Shi, Y. Zhong, L. Guo, and G. Li, Dalton Trans. 44, 4362 (2015).
G. Calleja, C. Martos, G. Orcajo, et al., CrystEngComm 17, 338 (2015).
B. Shi, Y. Zhong, L. Guo, and G. Li, Dalton Trans. 44, 4362 (2015).
J. Liu, ACS Catal. 7, 34 (2016).
C. J. Doonan and C. J. Sumby, CrystEngComm 19, 40448 (2017).
S. Goswami, G. Leitus, B. K. Tripuramallu, and I. Goldberg, Cryst. Growth Des. 17, 4393 (2017).
R. An, H. Zhao, H.M. Hu, et al., Inorg. Chem. 55, 871 (2016).
W. Huang, F.X. Shen, S.Q. Wu, et al., Inorg. Chem. 55, 5476 (2016).
Q. Q. Li, W. Q. Zhang, C. Y. Ren, et al., CrystEngComm 18, 3358 (2016).
S. S. Shen, X. F. Wang, H. M. Hu, et al., Polyhedron 91, 52 (2015).
M. Arici, O. Z. Yesilel, and M. Tas, Cryst. Growth Des. 15, 3024 (2015).
M. Du, C. P. Li, C. S. Liu, and S. M. Fang, Coord. Chem. Rev. 257, 1282 (2013).
M. Khalaj, A. Lalegani, J. Akbari, et al., J. Mol. Struct. 1169, 31 (2018).
A. Lalegani, M. Khaledi Sardashti, M. Khalaj, et al., Inorg. Chim. Acta 457, 136 (2017).
A. Lalegani, M. Khalaj, S. Sedaghat, et al., J. Mol. Struct. 1148, 479 (2017).
A Beheshti, A. Lalegani, G. Bruno, and H. Amiri Rudbari, J. Mol. Struct. 1071, 18 (2014).
H. Kon and T. Nagata, Inorg. Chem. 48, 8593 (2009).
R. Cao, P. Muller, and S. J. Lippard, J. Am. Chem. Soc. 132, 17366 (2010).
J. F. Song, R. S. Zhou, J. Zhang, and C. L. Pan, Z. Anorg, Allg. Chem. 638, 201 (2012).
C. Y. Li, L. Li, and T. L. Hu, J. Inorg. Organomet. Polym. 21, 682 (2011).
Q. Chen, M. H. Zeng, Y. L. Zhou, et al., Chem. Mater. 22, 2114 (2010).
R. M. Thomas, P. C. B. Widger, S. M. Ahmed, et al., J. Am. Chem. Soc. 132, 16520 (2010).
W. S. Tang, G. T. Wu, T. Liu, et al., J. Chem. Soc. Dalton Trans. 18, 2395 (2008).
S. Y. Zhang, Z. J. Zhang, W. Shi, et al., Inorg. Chim. Acta 363, 3787 (2010).
Y. Q. Wei, K. C. Wu, R. Broer, et al., Inorg. Chem. Commun. 10, 910 (2007).
J. F. Ma, J. F. Liu, Y. Xing, et al., Dalton Trans. 14, 2403 (2000).
Agilent, CrysAlis PRO (Agilent Technologies Ltd, Yarnton, Oxfordshire, 2014).
G. M. Sheldrick, Acta Crystallogr. C 71, 3 (2015).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009).
J. L. Lu, Y. S. Xue, J. Wei, and T. Zhou, Asian J. Chem. 29, 2643 (2017).
ACKNOWLEDGMENTS
The authors would like to acknowledge the Young Researchers and Elite Club, Buinzahra Branch, for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehdi Khalaj, Lalegani, A., Lyczko, K. et al. Synthesis and Characterization of Co(II) Coordination Polymer with a Flexible Bidentate Ligand. Crystallogr. Rep. 64, 1084–1088 (2019). https://doi.org/10.1134/S1063774519070071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774519070071