Abstract
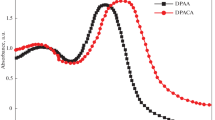
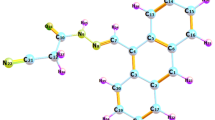
Metal-free organic compounds 24-SC ((E)-2-cyano-3-(2,4-dimethoxyphenyl)acrylic acid) and 34-SC ((E)-2-cyano-3-(3,4-dimethoxyphenyl)acrylic acid), containing methoxy groups as a donor and the acrylic acid as an acceptor were synthesized and characterized by CHN, FT-IR, UV-Vis, 1H-NMR and single crystal X-ray diffraction and used as photosensitizers for the application of dye-sensitized solar cells (DSSC). The sensitizing characteristics of them were evaluated. Both compounds contain the natural molecule, its anionic form and the piperidinium cation and they differ by number of these molecules in the asymmetric unit. To get further insight into the effect of molecular structure on the performance of DSSC, their geometry and energies of HOMO and LUMO were optimized by density functional theory calculation at the B3LYP/6-31G(d) level with Gaussian 03. Overall conversion efficiencies of 0.78 under full sunlight irradiation are obtained for DSSCs based on the new metal-free organic dyes 24-SC and 34-SC.
Similar content being viewed by others
References
M. Grätzel, Inorg. Chem. 44, 6841 (2005).
B. O’Regan and M. Grätzel, Nature 353, 737 (1991).
Ionic Liquids. basionics.com, November 2008.
M. Gratzel, Acc. Chem. Res. 42, 1788 (2009).
A. Hagfeldt, G. Boschloo, L. Sun, et al., Chem. Rev. 110, 6595 (2010).
C.-Y. Chen, J.-G. Chen, S.-J. Wu, et al., Angew. Chem. Int. Ed. 47, 7342 (2008).
Y. J. Chen and T. J. Chow, Tetrahedron 65, 4726 (2009).
Y.-S. Yen, H.-H. Chou, Y.-C. Chen, et al., J. Mater. Chem. 22, 8734 (2012).
M. Liang and J. Chen, Chem. Soc. Rev. 42, 3453 (2013).
A. Mishra, M. K. R. Fischer, and P. Bauerle, Angew. Chem. Int. Ed. 48, 2474 (2009).
L. Zhou, C. Jia, Z. Wan, et al., Dyes Pigm. 95, 743 (2015).
Y.-R. Gao, L.-L. Chu, W. Guo, and T.-L. Ma. Chin. Chem. Lett. 24, 149 (2013).
J. Shi, Z. Chai, C. Zhong, et al., Dyes Pigm. 95, 244 (2012).
L. Zhou, C. Jia, Z. Wan, et al., Org. Elect. 14, 1755 (2013).
Z. Iqbal, W.-Q. Wu, D.-B. Kuang, et al., Dyes Pigm. 96, 722 (2013).
Y.-D. Lin and T. J. Chow, J. Photochem. Photobiol. A: Chem. 230, 47 (2012).
Y.-D. Lin and T. J. Chow, J. Mater. Chem. 21, 14907 (2011).
S. Hwang, J. H. Lee, C. Park et al., Chem. Commun. 4887 (2007).
D. P. Hagberg, T. Edvinsson, T. Marinado, et al., Chem. Commun. 2245 (2006).
Y. Wu, M. Marszalek, S. M. Zakeeruddin, et al., Energy Environ. Sci. 5, 8261 (2012).
K.-H. Kim, S.-M. Lee, M.-H. Seo, et al., Macromol. Res. 20, 128 (2012).
D. H. Lee, M. J. Lee, H. M. Song, et al., Dyes Pigm. 91, 192 (2011).
Q. Feng, W. Zhang, G. Zhou, and Z.-S. Wang. Chem. Asian J. 8, 168 (2013).
T. Y. Wu, M. H. Tsao, F. L. Chen, et al., J. Iran. Chem. Soc. 7, 707 (2010).
H. Han, M. Liang, K. Tang, et al., J. Photochem. Photobiol. A: Chem. 225, 8 (2011).
S. Haid, M. Marszalek, A. Mishra, et al., Adv. Funct. Mater. 22, 1291 (2012).
H.-C. Chu, D. Sahu, Y.-C. Hsu, et al., Dyes Pigm. 93, 1488 (2012).
G.-W. Li, J. Xiao, and W.-Q. Zhang, Chin. Chem. Lett. 24, 52 (2013).
H. Ozawa, R. Shimizu, and H. Arakawa, RSC Adv. 2, 3198 (2012).
M. K. R. Fischer, S. Wenger, M. Wang, et al., Chem. Mater. 22, 1836 (2010).
J. Preat, C. Michaux, D. Jacquemin, and E. A. Perpete, J. Phys. Chem. C 113, 16821 (2009).
D. Kim, M.-S. Kang, K. Song, et al., Tetrahedron 64, 10417 (2008).
Y. J. Chang and T. J. Chow, J. Mater. Chem. 21, 9523 (2011).
Y. J. Chang, M. Watanabe, P.-T. Chou, and T. J. Chow, Chem. Commun. 48, 726 (2012).
Y. J. Chang, P.-T. Chou, Y. Z. Lin, et al., J. Mater. Chem. 22, 21704 (2012).
C.-J. Yang, Y. J. Chang, M. Watanabe, et al., J. Mater. Chem. 22, 4040 (2012).
Agilent Technologies. CrysAlisPRO (Yarnton, Oxford shire, UK, 2010).
L. Palatinus and G. Chapuis, J. Appl. Crystallogr. 40, 786 (2007).
V. Petricek, M. Dusek, and L. Palatinus, Z. Kristallogr. 229 (5), 345 (2014).
L. J. Farrugia, J. Appl. Crystallogr. 30, 656 (1997).
K. Brandenburg and H. Putz, DIAMOND Version 3 (Crystal ImpactGbR, Postfach 1251, D-53002 Bonn, Germany, 2005).
M. J. Frisch et al., GAUSSIAN 98, Revision A.9 (Gaussian, Pittsburgh, PA, 1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Khalaji, A.D., Maddahi, E., Dusek, M. et al. Organic compounds containing methoxy and cyanoacrylic acid: Synthesis, characterization, crystal structures, and theoretical studies. Crystallogr. Rep. 60, 1019–1026 (2015). https://doi.org/10.1134/S1063774515070123
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774515070123