Abstract
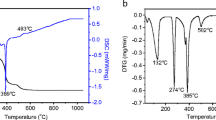
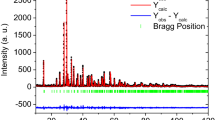
Polycrystalline Pr2Mo2O9 samples have been prepared by solid-state synthesis and single crystals of this compound have been grown. Pr2Mo2O9 is unstable in the temperature range 700–900°C and partially decomposes with the formation of Pr2Mo3O12 at these temperatures, but upon further heating to 1000–1050°C, Pr2Mo2O9 is recovered. At room temperature, the structure, polymorphism, and physical properties of Pr2Mo2O9 are similar to those of the known oxide ion conductor La2Mo2O9. Pr2Mo2O9 exhibits a reversible first-order phase transition to the cubic phase in the temperature range 520–540°C. The electric conductivity of Pr2Mo2O9 is close to that of La2Mo2O9 and amounts to 3.5 × 10−2 S/cm at 700°C. The conductivity of Pr2Mo2O9 is described by the Arrhenius law in the low-temperature phase and by the Vogel-Fulcher-Tammann equation in the high-temperature phase.
Similar content being viewed by others
References
P. Lacorre, F. Goutenoire, O. Bohnke, et al., Nature 404, 856 (2000).
E. Ya. Rode, G. V. Lysanova, and L. Z. Gokhman, Neorg. Mater. 7, 2101 (1971).
T. Yamazaki, T. Shimazaki, T. Hashizume, and K. Terayama, J. Mater. Sci. Lett. 21, 29 (2002).
G. Giable, L. Sandhya Kumari, V. S. Vishnu, et al., J. Solid State Chem. 181, 487 (2008).
J.-P. Fournier, J. Fournier, and R. Kohlmuller, Bull. Soc. Chim. Fr., p. 4277 (1970).
R. D. Shannon, Acta Crystallogr. A 32, 751 (1976).
L. A. Drobyshev, V. I. Ponomarev, I. T. Frolkina, and N. V. Belov, Sov. Phys. Crystallogr. 15, 391 (1970).
V. I. Voronkova, E. P. Kharitonova, A. E. Krasilnikova, and N. N. Kononkova, J. Phys.: Condens. Matter 20, 195210 (2008).
F. Goutenoire, O. Isnard, R. Retoux, and P. Lacorre, Chem. Mater. 12, 2575 (2000).
S. Georges, F. Goutenoire, O. Bohnke, et al., J. New Mater. Electrochem. Systems 7, 51 (2004).
C. Tealdi, G. Chiodelli, L. Malavasi, and G. Flor, J. Mater. Chem. 14, 3553 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of N.V. Belov
Original Russian Text © V.I. Voronkova, E.P. Kharitonova, E.I. Orlova, D.S. Kolesnikova, 2011, published in Kristallografiya, 2011, Vol. 56, No. 6, pp. 1135–1138.
Rights and permissions
About this article
Cite this article
Voronkova, V.I., Kharitonova, E.P., Orlova, E.I. et al. Synthesis and properties of oxide ion conductor Pr2Mo2O9 with La2Mo2O9 structure. Crystallogr. Rep. 56, 1066–1069 (2011). https://doi.org/10.1134/S1063774511060277
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774511060277