Abstract
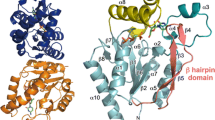
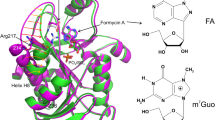
Uridine phosphorylase (UPh) belongs to pyrimidine nucleoside phosphorylases. This enzyme catalyzes cleavage of the C-N glycoside bond in uridine to form uracil and ribose-1’-phosphate. Uridine phosphorylase supplies cells with nucleotide precursors by catalyzing the phosphorolysis of purine and pyrimidine nucleosides. This is an alternative to de novo nucleotide synthesis. The three-dimensional structure of native uridine phosphorylase from Salmonella typhimurium (StUPh) in a new crystal form was solved and refined at 1.90 Å resolution (R st = 20.37%; R free = 24.69%; the rmsd of bond lengths and bond angles are 0.009 Å and 1.223°, respectively). A homodimer containing two asynchronously functioning active sites was demonstrated to be the minimum structural unit necessary for function of the hexameric StUPh molecule (L 33L 2). Each active site is formed by amino acid residues of both subunits.
Similar content being viewed by others
References
J. C. Leer, K. Hammer-Jespersen, and M. Schwarz, Eur. J. Biochem. 75, 217 (1977).
A. Vita, A. Amici, T. Cacciamani, et al., Int. J. Biochem. 18, 431 (1986).
D. S. Martin, R. L. Stolfi, and R. C. Sawyer, Cancer Chemother. Pharmacol. 24, 9 (1989).
M. Iigo, K. Nishikata, Y. Nakahiama, et al., Biochem. Pharmacol. 39, 1247 (1990).
A. Yoshimura, Y. Kuwazuru, T. Furukawa, et al., Biochim. Biophys. Acta. 1034, 107 (1990).
K. Usuki, J. Saras, J. Walenberger, et al., Biochem. Biophys. Res. Commun. 284, 1311 (1992).
C. Deliang, L. Rosalind, Russell, et al., Cancer Res. 62, 2313 (2002).
M. Zolotukhina, I. Ovcharova, S. Eremina, et al., Res. Microbiol. 154, 510 (2003).
O. K. Molchan, N. A. Dmitrieva, D. V. Romanova, et al., Biokhimiya 63, 235 (1998).
W. Kabsch, Appl. Crystallogr. 21, 219 (2001).
M. V. Dontsova, A. G. Gabdoulkhakov, O. K. Molchan, et al., Acta Crystallogr. F. 61, 337 (2005).
A. J. McCoy, R. W. Grosse-Kunstleve, L. C. Storoni, et al., Acta Crystallogr., Sect. D: Biol. Crystallogr. 61, 458 (2005).
T. A. Jones, J. Y. Zhou, S. W. Cowan, et al., Acta Crystallogr., Sect. A: Found. Crystallogr. 47, 110 (1991).
A. T. Brunger, P. D. Adams, G. M. Clore, et al., Acta Crystallogr., Sect. D: Biol. Crystallogr. 54, 905 (1998).
G. N. Murshudov, A. A. Vagin, and E. J. Dodson, Acta Crystallogr., Sect. D: Biol. Crystallogr. 53, 240 (1997).
P. Emsley and K. Cowtan Acta Crystallogr., Sect. D: Biol. Crystallogr. 60, 2126 (2004).
R. A. Laskowski, M. W. MacArthur, D. S. Moss, et al., Appl. Crystallogr. 26, 283 (1993).
F. T. Burling, R. Kniewel, J. A. Buglino, et al., Acta Crystallogr., Sect. D: Biol. Crystallogr. 59, 73 (2003).
C. Mao, W. J. Cook, M. Zhou, et al., Structure 5, 1373 (1997).
T. T. Caradoc-Davies, S. M. Cutfield, I. L. Lamont, et al., Mol. Biol. 337, 337 (2004).
M. R. Waltera, W. J. Cook, L. Brent Cole, et al., Biol. Chem. 265, 14016 (1990).
B. W. Matthews, Mol. Biol. 33, 491 (1968).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.I. Timofeev, B.F. Pavlyuk, A.A. Lashkov, T.A. Seregina, A.G. Gabdulkhakov, B.K. Vaĭnshteĭn, A.M. Mikhaĭlov, 2007, published in Kristallografiya, 2007, Vol. 52, No. 6, pp. 1106–1113.
Rights and permissions
About this article
Cite this article
Timofeev, V.I., Pavlyuk, B.F., Lashkov, A.A. et al. Structure of the homodimer of uridine phosphorylase from Salmonella typhimurium in the native state at 1.9 Å resolution. Crystallogr. Rep. 52, 1072–1078 (2007). https://doi.org/10.1134/S1063774507060235
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1063774507060235