Abstract—
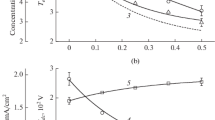
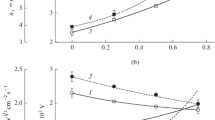
The electrophysical parameters of plasma and the kinetics of active particles in CF4 + Ar and CHF3 + Ar mixtures under induction RF (13.56 MHz) discharge are compared. It is shown that the CHF3 + Ar system containing 0–75% Ar is intrinsic for systematically lower concentrations and flow densities of fluorine atoms, while they are higher in the case of fluorocarbon radicals and positive ions. The set of formal parameters in the form of flux density ratios is suggested in order to describe the formation and destruction of a fluorocarbon polymer film. It is confirmed that the advantage of the CHF3 + Ar system according to the etching selectivity of SiO2/Si is caused by its higher polymerization ability.



Similar content being viewed by others
REFERENCES
Wolf, S. and Tauber, R.N., Silicon Processing for the VLSI Era, Vol. 1: Process Technology, New York: Lattice, 2000.
Handbook of Plasma Processing Technology, Rossnagel, S.M., Cuomo, J.J., and Westwood, W.D., Eds., Park Ridge: Noyes, 1990.
Roosmalen, A.J., Baggerman, J.A.G., and Brader, S.J.H., Dry Etching for VLSI, New York: Plenum, 1991.
Kimura, T. and Ohe, K., Model and probe measurements of inductively coupled CF4 discharges, J. Appl. Phys., 2002, vol. 92, pp. 1780–1787.
Kimura, T. and Ohe, K., Probe measurements and global model of inductively coupled Ar/CF4 discharges, Plasma Sources Sci. Technol., 1999, vol. 8, pp. 553–560.
Standaert, T.E.F.M., Hedlund, C., Joseph, E.A., and Oehrlein, G.S., Role of fluorocarbon film formation in the etching of silicon, silicondioxide, silicon nitride, and amorphous hydrogenated silicon carbide, J. Vac. Sci. Technol., A, 2004, vol. 22, pp. 53–60.
Lee, H.K., Chung, K.S., and Yu, J.S., Selective etching of thick Si3N4, SiO2 and Si by using CF4/O2 and C2F6 gases with or without O2 or Ar addition, J. Korean Phys. Soc., 2009, vol. 54, pp. 1816–1824.
Lieberman, M.A. and Lichtenberg, A.J., Principles of Plasma Discharges and Materials Processing, New York: Wiley, 1994.
Yeom, G.Y. and Kushner, M.J., Si/SiO2 etch properties using CF4 and CHF3 in radio frequency cylindrical magnetron discharges, Appl. Phys. Lett., 1990, vol. 56, pp. 857–859.
Gaboriau, F., Cartry, G., Peignon, M.-C., and Cardinaud, Ch., Selective and deep plasma etching of SiO2: comparison between different fluorocarbon gases (CF4, C2F6, CHF3) mixed with CH4 or H2 and influence of the residence time, J. Vac. Sci. Technol., B, 2002, vol. 20, pp. 1514–1521.
Ho, P., Johannes, J.E., and Buss, R.J., Modeling the plasma chemistry of C2F6 and CHF3 etching of silicon dioxide, with comparisons to etch rate and diagnostic data, J. Vac. Sci. Technol., A, 2001, vol. 19, pp. 2344–2367.
Bose, D., Rao, M.V.V.S., Govindan, T.R., and Meyyappan, M., Uncertainty and sensitivity analysis of gas-phase chemistry in a CHF3 plasma, Plasma Sources Sci. Technol., 2003, vol. 12, pp. 225–234.
Proshina, O., Rakhimova, T.V., Zotovich, A., Lopaev, D.V., Zyryanov, S.M., and Rakhimov, A.T., Multifold study of volume plasma chemistry in Ar/CF4 and Ar/CHF3 CCP discharges, Plasma Sources Sci. Technol., 2017, in press. https://doi.org/10.1088/1361-6595/aa72c9.
Chun, L., Efremov, A., Yeom, G.Y., and Kwon, K.-H., A comparative study of CF4/O2/Ar and C4F8/O2/Ar plasmas for dry etching applications, Thin Solid Films, 2015, vol. 579, pp. 136–148.
Son, J., Efremov, A., Yun, S.J., Yeom, G.Y., and Kwon, K.-H., Etching characteristics and mechanism of sinxfilms for nano-devices in CH2F2/O2/Ar inductively coupled plasma: effect of O2 mixing ratio, J. Nanosci. Nanotech., 2014, vol. 14, pp. 9534–9540.
Johnson, E.O. and Malter, L., A floating double probe method for measurements in gas discharges, Phys. Rev., 1950, vol. 80, pp. 58–70.
Sugavara, M., Plasma Etching: Fundamentals and Applications, New York: Oxford Univ. Press, 1998.
Kwon, K.-H., Efremov, A., Kim, M., Min, N.K., Jeong, J., and Kim, K., A model-based analysis of plasma parameters and composition in HBr/X (X = Ar, Ge, N2) inductively coupled plasmas, J. Electrochem. Soc., 2010, vol. 157, pp. H574–H579.
Efremov, A., Min, N.K., Choi, B.G., Baek, K.H., and Kwon, K.-H., Model-based analysis of plasma parameters and active species kinetics in Cl2/X (X = Ar, He, N2) inductively coupled plasmas, J. Electrochem. Soc., 2008, vol. 155, pp. D777–D782.
Kokkoris, G., Goodyear, A., Cooke, M., and Gogolides, E., A global model for C4F8 plasmas coupling gas phase and wall surface reaction kinetics, J. Phys. D: Appl. Phys., 2008, vol. 41, p. 195211.
NIST Chemical Kinetics Database. https://kinetics. nist.gov/kinetics/welcome.jsp.
Efremov, A.M., Kim, D.-P., and Kim, C.-I., Effect of gas mixing ratio on gas-phase composition and etch rate in an inductively coupled CF4/Ar plasma, Vacuum, 2004, vol. 75, pp. 133–142.
Lele, C., Liang, Z., Linda, X., Dongxia, L., Hui, C., and Tod, P., Role of CF2 in the etching of SiO2, Si3N4 and Si in fluorocarbon plasma, J. Semicond., 2009, vol. 30, p. 033005-1.
Kay, E., Coburn, J., and Dilks, A., Plasma chemistry of fluorocarbons as related to plasma etching and plasma polymerization, in Plasma Chemistry III. Topics in Current Chemistry, Eds. by S. Veprek and M. Venugopalan (Springer, Berlin, 1980), vol. 94, pp. 123–145.
Kay, E. and Dilks, A., Plasma polymerization of fluorocarbons in rf capacitively coupled diode system, J. Vac. Sci. Technol., 1981, vol. 18, pp. 1–11.
Stoffels, W.W., Stoffels, E., and Tachibana, K., Polymerization of fluorocarbons in reactive ion etching plasmas, J. Vac. Sci. Technol., A, 1998, vol. 16, pp. 87–95.
Gray, D.C., Tepermeister, I. and Sawin, H.H., Phenomenological modeling of ion enhanced surface kinetics in fluorine-based plasma etching, J. Vac. Technol., B, 1993, vol. 11, pp. 1243–1257.
Efremov, A.M., Kim, D.P., and Kim, C.-I., Simple model for ion-assisted etching using Cl2/Ar inductively coupled plasma: effect of gas mixing ratio, IEEE Trans. Plasma Sci., 2004, vol. 32, pp. 1344–1351.
Jansen, H., Gardeniers, H., de Boer, M., Elwenspoek, M., and Fluitman, J., A survey on the reactive ion etching of silicon in microtechnology, J. Micromech. Microeng., 1996, vol. 6, pp. 14–28.
ACKNOWLEDGMENTS
The work was supported by the Russian Foundation for Basic Research (project no. 18-37-00064 mol_а).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Muravev
Rights and permissions
About this article
Cite this article
Efremov, A.M., Murin, D.B. & Kwon, KH. Parameters of Plasma and Kinetics of Active Particles in CF4 (CHF3) + Ar Mixtures of a Variable Initial Composition. Russ Microelectron 47, 371–380 (2018). https://doi.org/10.1134/S1063739718060033
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063739718060033