Abstract
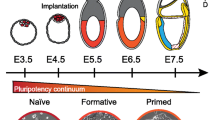
The early embryonic development of mice and humans is characterized by the presence of pluripotent cells, which give rise to all tissues of the developing embryo. About 40 years ago, these cells were isolated and maintained in culture in a stable pluripotent state. Since then, a plethora of data regarding the mechanisms underlying the functioning of these cells, key genes necessary for their work, as well as their differentiation into various cell types. Nowadays, depending on the stage of embryogenesis, multiple types of pluripotent stem cells can be distinguished, and these types fundamentally differ from each other in signaling, culture condition dependendence, and differentiation abilities. This review summarizes currently available information on the dynamics of pluripotency during embryogenesis as well as in cultured pluripotent cells.



Similar content being viewed by others
REFERENCES
Azami, T., Matsumoto, K., Jeon, H., et al., Klf5 suppresses ERK signaling in mouse pluripotent stem cells, PLoS One, 2018, vol. 13, no. 11, art. e0207321.
Barral, A., Rollan, I., Sanchez-Iranzo, H., et al., Nanog regulates Pou3f1 expression at the exit from pluripotency during gastrulation, Biol. Open, 2019, vol. 8, no. 11, art. bio046367.
Bayerl, J., Ayyash, M., Shani, T., et al., Principles of signaling pathway modulation for enhancing human naive pluripotency induction, Cell Stem Cell, 2021, vol. 28, pp. 1–17.
Bedzhov, I., Bialecka, M., Zielinska, A., et al., Development of the anterior-posterior axis is a self-organizing process in the absence of maternal cues in the mouse embryo, Cell Res., 2015, vol. 25, no. 12, pp. 1368–1371.
Ben-Haim, N., Lu, C., Guzman-Ayala, M., et al., The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4, Dev. Cell, 2006, vol. 11, no. 3, pp. 313–323.
Bessonnard, S., De Mot, L., Gonze, D., et al., Gata6, Nanog and Erk signaling control cell fate in the inner cell mass through a tristable regulatory network, Development, 2014, vol. 141, no. 19, pp. 3637–3648.
Blanco, E., González-Ramírez, M., Alcaine-Colet, A., et al., The bivalent genome: characterization, structure, and regulation, Trends Genet., 2020, vol. 36, no. 2, pp. 118–131.
Boroviak, T., Loos, R., Bertone, P., et al., The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification, Nat. Cell Biol., 2014, vol. 16, no. 6, pp. 513–525.
Brons, I.G.M., Smithers, L.E., Trotter, M.W.B., et al., Derivation of pluripotent epiblast stem cells from mammalian embryos, Nature, 2007, vol. 448, no. 7150, pp. 191–195.
Buecker, C., Srinivasan, R., Wu, Z., et al., Reorganization of enhancer patterns in transition from naive to primed pluripotency, Cell Stem Cell, 2014, vol. 14, no. 6, pp. 838–853.
Cajal, M., Lawson, K.A., Hill, B., et al., Clonal and molecular analysis of the prospective anterior neural boundary in the mouse embryo, Development, 2012, vol. 139, no. 2, pp. 423–436.
Chambers, I., Colby, D., Robertson, M., et al., Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells, Cell, 2003, vol. 113, no. 5, pp. 643–655.
Chambers, I., Silva, J., Colby, D., et al., Nanog safeguards pluripotency and mediates germline development, Nature, 2007, vol. 450, no. 7173, pp. 1230–1234.
Chan, Y.S., Göke, J., Ng, J.H., et al., Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast, Cell Stem Cell, 2013, vol. 13, no. 6, pp. 663–675.
Chhabra, S., Liu, L., Goh, R., et al., Dissecting the dynamics of signaling events in the BMP, WNT, and NODAL cascade during self-organized fate patterning in human gastruloids, PLoS Biol., 2019, vol. 17, no. 10, art. e3000498.
Choi, H. W., Joo, J.Y., Hong, Y.J., et al., Distinct enhancer activity of Oct4 in naive and primed mouse pluripotency, Stem Cell Rep., 2016, vol. 7, no. 5, pp. 911–926.
Costello, I., Pimeisl, I.M., Dräger, S., et al., The T-box transcription factor eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation, Nat. Cell Biol., 2011, vol. 13, no. 9, pp. 1084–1091.
Cruz-Molina, S., Respuela, P., Tebartz, C., et al., Prc2 facilitates the regulatory topology required for poised enhancer function during pluripotent stem cell differentiation, Cell Stem Cell, 2017, vol. 20, no. 5, pp. 689–705.
DeVeale, B., Brokhman, I., Mohseni, P., et al., Oct4 is required ~E7.5 for proliferation in the primitive streak, PLoS Genet., 2013, vol. 9, no. 11.
Evans, M.J. and Kaufman, M.H., Establishment in culture of pluripotential cells from mouse embryos, Nature, 1981, vol. 292, no. 5819, pp. 154–156.
Factor, D.C., Corradin, O., Zentner, G.E., et al., Epigenomic comparison reveals activation of “seed” enhancers during transition from naive to primed pluripotency, Cell Stem Cell, 2014, vol. 14, no. 6, pp. 854–863.
Ferretti, E. and Hadjantonakis, A.K., Mesoderm specification and diversification: from single cells to emergent tissues, Curr. Opin. Cell Biol., 2019, vol. 61, pp. 110–116.
Francou, A. and Anderson, K.V., The epithelial-to-mesenchymal transition in development and cancer, Annu. Rev. Cancer Biol., 2020, vol. 4, pp. 197–220.
Grosswendt, S., Kretzmer, H., Smith, Z.D., et al., Epigenetic regulator function through mouse gastrulation, Nature, 2020, vol. 584, no. 7819, pp. 102–108.
Guo, Q. and Li, J.Y.H., Distinct functions of the major Fgf8 spliceform, before and during mouse gastrulation, Development, 2007, vol. 134, no. 12, pp. 2251–2260.
Guo, G., Pinello, L., Han, X., et al., Serum-based culture conditions provoke gene expression variability in mouse embryonic stem cells as revealed by single-cell analysis, Cell Rep., 2016, vol. 14, no. 4, pp. 956–965.
Habibi, E., Brinkman, A.B., Arand, J., et al., Whole-genome bisulfite sequencing of two distinct interconvertible DNA methylomes of mouse embryonic stem cells, Cell Stem Cell, 2013, vol. 13, no. 3, pp. 360–369.
Hayashi, K., Lopes, S.M.C., Tang, F., et al., Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states, Cell Stem Cell, 2008, vol. 3, no. 4, pp. 391–401.
Hayashi, K., Ohta, H., Kurimoto, K., et al., Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells, Cell, 2011, vol. 146, no. 4, pp. 519–532.
Huang, Y., Osorno, R., Tsakiridis, A., et al., In vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation, Cell Rep., 2012, vol. 2, no. 6, pp. 1571–1578.
Ivanova, N., Dobrin, R., Lu, R., et al., Dissecting self-renewal in stem cells with rna interference, Nature, 2006, vol. 442, no. 7102, pp. 533–538.
Kimura-Yoshida, C., Nakano, H., Okamura, D., et al., Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm, Dev. Cell, 2005, vol. 9, no. 5, pp. 639–650.
Kinoshita, M., Barber, M., Mansfield, W., et al., Capture of mouse and human stem cells with features of formative pluripotency, Cell Stem Cell, 2021, vol. 28, no. 3, pp. 453–471.
Koch, F., Scholze, M., Wittler, L., et al., Antagonistic activities of Sox2 and Brachyury control the fate choice of neuro-mesodermal progenitors, Dev. Cell, 2017, vol. 42, no. 5, pp. 514–526.
Kojima, Y., Kaufman-Francis, K., Studdert, J.B., et al., The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak, Cell Stem Cell, 2014, vol. 14, no. 1, pp. 107–120.
Kopp, J.L., Ormsbee, B.D., Desler, M., et al., Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells, Stem Cells, 2008, vol. 26, no. 4, pp. 903–911.
Kwon, G.S., Viotti, M., and Hadjantonakis, A.K., The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages, Dev. Cell, 2008, vol. 15, no. 4, pp. 509–520.
Liu, C., Wang, R., He, Z., et al., Suppressing nodal signaling activity predisposes ectodermal differentiation of epiblast stem cells, Stem Cell Rep., 2018, vol. 11, no. 1, pp. 43–57.
Malleshaiah, M., Padi, M., Rue, P., et al., Nac1 coordinates a sub-network of pluripotency factors to regulate embryonic stem cell differentiation, Cell Rep., 2016, vol. 14, no. 5, pp. 1181–1194.
Martin, G.R., Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells, Proc. Natl. Acad. Sci. U. S. A., 1981, vol. 78, no. 12, part II, pp. 7634–7638.
Martin Gonzalez, J., Morgani, S.M., Bone, R.A., et al., Embryonic stem cell culture conditions support distinct states associated with different developmental stages and potency, Stem Cell Rep., 2016, vol. 7, no. 2, pp. 177–191.
Martyn, I., Siggia, E.D., and Brivanlou, A.H., Mapping cell migrations and fates in a gastruloid model to the human primitive streak, Curr. Opin. Cell Biol., 2019, vol. 66, pp. 89–96.
Mas, G., Blanco, E., Ballaré, C., et al., Promoter bivalency favors an open chromatin architecture in embryonic stem cells, Nat. Genet., 2018, vol. 50, no. 10, pp. 1452–1462.
Masui, S., Nakatake, Y., Toyooka, Y., et al., Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells, Nat. Cell Biol., 2007, vol. 9, no. 6, pp. 625–635.
Meyenn, F. von, Iurlaro, M., Habibi, E., et al., Impairment of DNA methylation maintenance is the main cause of global demethylation in naive embryonic stem cells, Mol. Cell, 2016, vol. 62, no. 6, pp. 848–861.
Mittnenzweig, M., Mayshar, Y., Cheng, S., et al., Article a single-embryo, single-cell time-resolved model for mouse gastrulation a single-embryo, single-cell time-resolved model for mouse gastrulation, Cell, 2021, vol. 184, no. 11, pp. 2825–2842. e22.
Mohammed, H., Hernando-Herraez, I., Savino, A., et al., Single-cell landscape of transcriptional heterogeneity and cell fate decisions during mouse early gastrulation, Cell Rep., 2017, vol. 20, no. 5, pp. 1215–1228.
Morgani, S., Nichols, J., and Hadjantonakis, A.K., The many faces of pluripotency: in vitro adaptations of a continuum of in vivo states, BMC Dev. Biol., 2017, vol. 17, no. 1, pp. 1–20.
Morgani, S.M., Metzger, J.J., Nichols, J., et al., Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning, Elife, 2018, vol. 7, no. 7, art. e32839.
Mulas, C., Chia, G., Jones, K.A., et al., Oct4 regulates the embryonic axis and coordinates exit from pluripotency and germ layer specification in the mouse embryo, Development, 2018, vol. 145, no. 12, pp. 1–13.
Neagu, A., van Genderen, E., Escudero, I., et al., In vitro capture and characterization of embryonic rosette-stage pluripotency between naive and primed states, Nat. Cell Biol., 2020, vol. 22, no. 5, pp. 534–545.
Niwa, H., Miyazaki, J., and Smith, A.G., Quantitative expression of oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells, Nat. Genet., 2000, vol. 24, no. 4, pp. 372–376.
Niwa, H., Ogawa, K., Shimosato, D., et al., A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells, Nature, 2009, vol. 460, no. 7251, pp. 118–122.
Nowotschin, S., Setty, M., Kuo, Y.Y., et al., The emergent landscape of the mouse gut endoderm at single-cell resolution, Nature, 2019, vol. 569, no. 7756, pp. 361–367.
Ohinata, Y., Ohta, H., Shigeta, M., et al., A signaling principle for the specification of the germ cell lineage in mice, Cell, 2009, vol. 137, no. 3, pp. 571–584.
Perea-Gomez, A., Vella, F.D.J., Shawlot, W., et al., Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks, Dev. Cell, 2002, vol. 3, no. 5, pp. 745–756.
Pijuan-Sala, B., Griffiths, J.A., Guibentif, C., et al., A single-cell molecular map of mouse gastrulation and early organogenesis, Nature, 2019, vol. 566, no. 7745, pp. 490–495.
Radzisheuskaya, A., Chia Gle.B., dos Santos, R.L., et al., A defined Oct4 level governs cell state transitions of pluripotency entry and differentiation into all embryonic lineages, Nat. Cell Biol., 2013, vol. 15, no. 6, pp. 579–590.
Rodriguez, T.A., Srinivas, S., Clements, M.P., et al., Induction and migration of the anterior visceral endoderm is regulated by the extra-embryonic ectoderm, Development, 2005, vol. 132, no. 11, pp. 2513–2520.
Saykali, B., Mathiah, N., Nahaboo, W., et al., Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo, Elife, 2019, vol. 8, art. e42434.
Shahbazi, M.N., Scialdone, A., Skorupska, N., et al., Pluripotent state transitions coordinate morphogenesis in mouse and human embryos, Nature, 2017, vol. 552, pp. 239–243.
Smith, A., Formative pluripotency: the executive phase in a developmental continuum, Development, 2017, vol. 144, no. 3, pp. 365–373.
Sozen, B., Cox, A.L., De Jonghe, J., et al., Self-organization of mouse stem cells into an extended potential blastoid, Dev. Cell, 2019, vol. 51, no. 6, pp. 698–712.
Srinivas, S., Rodriguez, T., Clements, M., et al., Active cell migration drives the unilateral movements of the anterior visceral endoderm, Development, 2004, vol. 131, no. 5, pp. 1157–1164.
Takaoka, K., Yamamoto, M., Shiratori, H., et al., The mouse embryo autonomously acquires anterior-posterior polarity at implantation, Dev. Cell, 2006, vol. 10, no. 4, pp. 451–459.
Tee, W.W., Shen, S.S., Oksuz, O., et al., Erk1/2 activity promotes chromatin features and RNAPII phosphorylation at developmental promoters in mouse ESCs, Cell, 2014, vol. 156, no. 4, pp. 678–690.
Teo, A.K.K., Arnold, S.J., Trotter, M.W.B., et al., Pluripotency factors regulate definitive endoderm specification through eomesodermin, Genes Dev., 2011, vol. 25, no. 3, pp. 238–250.
Tesar, P.J., Chenoweth, J.G., Brook, F.A., et al., New cell lines from mouse epiblast share defining features with human embryonic stem cells, Nature, 2007, vol. 448, no. 7150, pp. 196–199.
Theunissen, T.W., Friedli, M., He, Y., et al., Molecular criteria for defining the naive human pluripotent state, Cell Stem Cell, 2016, vol. 19, no. 4, pp. 502–515.
Thomson, J.A., Itskovitz-Eldor, J., Shapiro, S.S., et al., Embryonic stem cell lines derived from human blastocysts, Science, 1998, vol. 282, no. 5391, pp. 1145–1147.
Thomson, M., Liu, S.J., Zou, L.N., et al., Pluripotency factors in embryonic stem cells regulate differentiation into germ layers, Cell, 2011, vol. 14, no. 6, pp. 875–889.
Tortelote, G.G., Hernández-Hernández, J.M., Quaresma, A.J.C., et al., Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice, Dev. Biol., 2013, vol. 374, no. 1, pp. 164–173.
Viotti, M., Nowotschin, S., and Hadjantonakis, A.K., SOX17 links gut endoderm morphogenesis and germ layer segregation, Nat. Cell Biol., 2014, vol. 16, no. 12, pp. 1146–1156.
Wang, Z., Oron, E., Nelson, B., et al., Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells, Cell Stem Cell, 2012, vol. 10, no. 4, pp. 440–454.
Wang, R.N., Green, J., Wang, Z., et al., Bone morphogenetic protein (BMP) signaling in development and human diseases, Genes Dis., 2014, vol. 1, no. 1, pp. 87–105.
Wu, J., Okamura, D., Li, M., et al., An alternative pluripotent state confers interspecies chimaeric competency, Nature, 2015, vol. 521, no. 7552, pp. 316–321.
Yang, S.H., Kalkan, T., Morissroe, C., et al., Otx2 and Oct4 drive early enhancer activation during embryonic stem cell transition from naive pluripotency, Cell Rep., 2014, vol. 7, no. 6, pp. 1968–1981.
Yang, J., Ryan, D.J., Wang, W., et al., Establishment of mouse expanded potential stem cells, Nature, 2017, vol. 550, no. 7676, pp. 393–397.
Yeom, Y.Il., Fuhrmann, G., Ovitt, C.E., et al., Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells, Development, 1996, vol. 122, no. 3, pp. 881–894.
Yi, F., Pereira, L., Hoffman, J.A., et al., Opposing effects of Tcf3 and Tcf1 control wnt stimulation of embryonic stem cell self-renewal, Nat. Cell Biol., 2011, vol. 13, no. 7, pp. 762–770.
Ying, Q.L., Wray, J., Nichols, J., et al., The ground state of embryonic stem cell self-renewal, Nature, 2008, vol. 453, no. 7194, pp. 519–523.
Yu, Y., Wang, X., Zhang, X., et al., ERK inhibition promotes neuroectodermal precursor commitment by blocking self-renewal and primitive streak formation of the epiblast, Stem Cell Res. Ther., 2018, vol. 9, no. 1, p. 2.
Yu, L., Wei, Y., Sun, H.X., et al., Derivation of intermediate pluripotent stem cells amenable to primordial germ cell specification, Cell Stem Cell, 2021, vol. 28, no. 3, pp. 550–567. e12.
Zalc, A., Sinha, R., Gulati, G.S., et al., Reactivation of the pluripotency program precedes formation of the cranial neural crest, Science, 2021, vol. 371, no. 6529.
Zhang, M., Leitch, H.G., Tang, W.W.C., et al., Esrrb complementation rescues development of Nanog-null germ cells, Cell Rep., 2018, vol. 22, no. 2, pp. 332–339.
Zhang, S., Bell, E., Zhi, H., et al., OCT4 and PAX6 determine the dual function of SOX2 in human ESCs as a key pluripotent or neural factor, Stem Cell Res. Ther., 2019, vol. 10, no. 1, pp. 1–14.
Funding
The work was performed with the financial support of the grant of the Russian Science Foundation, no. 20-74-00072.
Author information
Authors and Affiliations
Contributions
Gordeev M.N. wrote the main part of the text, analyzed literature, and prepared illustrations. Bakhmet E.I. analyzed the literature, wrote an introduction and conclusion, and edited the text. Tomilin A. N. edited the text and made final edits.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests. This article does not contain any studies involving human participants or laboratory animals as experimental models performed by the authors.
Additional information
Translated by A. Ermakov
Rights and permissions
About this article
Cite this article
Gordeev, M.N., Bakhmet, E.I. & Tomilin, A.N. Pluripotency Dynamics during Embryogenesis and in Cell Culture. Russ J Dev Biol 52, 379–389 (2021). https://doi.org/10.1134/S1062360421060059
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360421060059