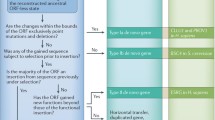
Abstract
Evolutionary biologists have always been interested in the origin and evolution of new genes. The most obvious mechanisms of their formation are various types of chromosomal and intergenic rearrangements, suggesting the use of existing genes as the source material. The possibility of the origin of a fully functional gene from noncoding DNA, i.e., de novo, was not rejected but it was practically considered as impossible until recently. Nevertheless, in 1996, after analysis of the yeast Saccharomyces cerevisiae genome, the first experimental evidence of the possibility of de novo gene formation was obtained. Ten years later, genes that do not have homologs, presumably having originated de novo, were found in Drosophila. The relatively high probability of the occurrence of genes de novo, estimated in bioinformatics studies, raised the interest in this topic and made the search for genes of this type relevant. Recently, the number of works devoted to the problem of the emergence of de novo genes in different organisms, including humans, is constantly growing, demystifying this phenomenon. Nevertheless, there are still many questions that require theoretical and practical research. This review is devoted to the problem of finding and characterizing genes that have arisen de novo, as well as to the proposed mechanisms of their occurrence.
Similar content being viewed by others
REFERENCES
Abdulrehman, D., Monteiro, P.T., Teixeira, M.C., et al., YEASTRACT: providing a programmatic access to curated transcriptional regulatory associations in Saccharomyces cerevisiae through a web services interface, Nucleic Acids Res., 2011, vol. 39, pp. D136–D140.
Abrusán, G., Integration of new genes into cellular networks, and their structural maturation, Genetics, 2013, vol. 195, no. 4, pp. 1407–1417.
Altschul, S.F., Gish, W., Miller, W., et al., Basic local alignment search tool, J. Mol. Biol., 1990, vol. 215, no. 3, pp. 403–410.
Altschul, S.F., Madden, T.L., Schaffer, A.A., et al., Gapped BLAST and PSI-BLAST: a new generation of protein database search programs, Nucleic Acids Res., 1997, vol. 25, no. 17, pp. 3389–3402.
Andersson, D.I., Jerlström-Hultqvist, J., and Nasvall, J., Evolution of new functions de novo and from preexisting genes, Cold Spring Harb. Perspect. Biol., 2015, vol. 7, no. 6, art. a017996.
Ángyán, A.F., Perczel, A., and Gáspári, Z., Estimating intrinsic structural preferences of de novo emerging random-sequence proteins: is aggregation the main bottleneck?, FEBS Lett., 2012, vol. 586, no. 16, pp. 2468–2472.
Basile, W., Sachenkova, O., Light, S., et al., High GC content causes orphan proteins to be intrinsically disordered, PLoS Comp. Biol., 2017, vol. 13, no. 3, art. e1005375.
Begun, D.J., Lindfors, H.A., Thompson, M.E., et al., Recently evolved genes identified from Drosophila yakuba and D. erecta accessory gland expressed sequence tags, Genetics, 2006, vol. 172, pp. 1675–1681.
Begun, D.J., Lindfors, H.A., Kern, A.D., et al., Evidence for de novo evolution of testis-expressed genes in the Drosophila yakuba/Drosophila erecta clade, Genetics, 2007, vol. 176, pp. 1131–1137.
Bekpen, C., Xie, C., and Tautz, D., Dealing with the adaptive immune system during de novo evolution of genes from intergenic sequences, BMC Evol. Biol., 2018, vol. 18.
Bitard-Feildel, T., Heberlein, M., Bornberg-Bauer, E., et al., Detection of orphan domains in Drosophila using “hydrophobic cluster analysis,” Biochimie, 2015, vol. 119, pp. 244–253.
Cai, J., Zhao, R., Jiang, H., et al., De novo origination of a new protein-coding gene in Saccharomyces cerevisiae, Genetics, 2008, vol. 179, no. 1, pp. 487–496.
Carelli, F.N., Liechti, A., Halbert, J., et al., Repurposing of promoters and enhancers during mammalian evolution, Nat. Commun., 2018, vol. 9, no. 1, p. 4066.
Carvunis, A.-R., Rolland, T., Wapinski, I., et al., Proto-genes and de novo gene birth, Nature, 2012, vol. 487, pp. 370–374.
Casola, C., From de novo to “de nono:” the majority of novel protein-coding genes identified with phylostratigraphy are old genes or recent duplicates, Genome Biol. Evol., 2018, vol. 10, no. 11, pp. 2906–2918.
Chen, S., Zhang, Y.E., and Long, M., New genes in Drosophila quickly become essential, Science, 2010, vol. 330, pp. 1682–1685.
Chen, S., Krinsky, B.H., and Long, M., New genes as drivers of phenotypic evolution, Nat. Rev. Genet., 2013, vol. 14, no. 9, pp. 645–660.
Clark, N.L., Aagaard, J.E., and Swanson, W.J., Evolution of reproductive proteins from animals and plants, Reproduction, 2006, vol. 131, no. 1, pp. 11–22.
Clark, M.B., Amaral, P.P., Schlesinger, F.J., et al., The reality of pervasive transcription, PLoS Biol., 2011, vol. 9, no. 7, art. e1000625.
Costanzo, M., Baryshnikova, A., Bellay, J., et al., The genetic landscape of a cell, Science, 2010, vol. 327, no. 5964, pp. 425–431.
Domazet-Lošo, T. and Tautz, D., A phylogenetically based transcriptome age index mirrors ontogenetic divergence patterns, Nature, 2010, vol. 468, no. 7325, pp. 815–818.
Domazet-Lošo, T., Brajković, J., and Tautz, D., A phylostratigraphy approach to uncover the genomic history of major adaptations in metazoan lineages, Trends Genet., 2007, vol. 23, no. 11, pp. 533–539.
Donoghue, M.T., Keshavaiah, C., Swamidatta, S.H., et al., Evolutionary origins of brassicaceae specific genes in Arabidopsis thaliana, BMC Evol. Biol., 2011, vol. 11, no. 1, p. 47.
Doolittle, W.F., Brunet, T.D.P., Linquist, S., et al., Distinguishing between “function” and “effect” in genome biology, Genome Biol. Evol., 2014, vol. 6, no. 5, pp. 1234–1237.
Dowling, D., Schmitz, J.F., and Bornberg-Bauer, E., Stochastic gain and loss of novel transcribed open reading frames in the human lineage, Genome Biol. Evol., 2020, vol. 12, no. 11, pp. 2183–2195.
Dujon, B., The yeast genome project: what did we learn?, Trends Genet., 1996, vol. 12, no. 7, pp. 263–270.
Ekman, D. and Elofsson, A., Identifying and quantifying orphan protein sequences in fungi, J. Mol. Biol., 2010, vol. 396, no. 2, pp. 396–405.
Elhaik, E., Sabath, N., and Graur, D., The “inverse relationship between evolutionary rate and age of mammalian genes” is an artifact of increased genetic distance with rate of evolution and time of divergence, Mol. Biol. Evol., 2006, vol. 23, no. 1, pp. 1–3.
Espinar, L., Tamarit, M.A.S., Domingo, J., and Carey, L.B., Promoter architecture determines cotranslational regulation of mRNA, Genome Res., 2018, vol. 28, no. 4, pp. 509–518.
Gehrmann, T. and Reinders, M.J.T., Proteny: discovering and visualizing statistically significant syntenic clusters at the proteome level, Bioinformatics, 2015, vol. 31, no. 21, pp. 3437–3444.
Ghiurcuta, C.G. and Moret, B.M.E., Evaluating synteny for improved comparative studies, Bioinformatics, 2014, vol. 30, no. 12, pp. i9–i18.
Grasse, P.-P., Evolution of Living Organisms, London: Academic, 1977.
Guerzoni, D. and McLysaght, A., De novo genes arise at a slow but steady rate along the primate lineage and have been subject to incomplete lineage sorting, Genome Biol. Evol., 2016, vol. 8, no. 4, pp. 1222–1232.
Guo, W.-J., Li, P., Ling, J., et al., Significant comparative characteristics between orphan and nonorphan genes in the rice (Oryza sativa L.) genome, Comp. Func. Genomics, 2007, vol. 2007, p. 21676.
Haberle, V. and Stark, A., Eukaryotic core promoters and the functional basis of transcription initiation, Nat. Rev. Mol., 2018, vol. 19, no. 10, pp. 621–637.
Haldane, J.B.S., The part played by recurrent mutation in evolution, Am. Nat., 1933, vol. 67, no. 708, pp. 5–19.
He, B., Chen, C., Teng, L., et al., Global view of enhancer-promoter interactome in human cells, Proc. Natl. Acad. Sci. U. S. A., 2014, vol. 111, no. 21, pp. E2191–E2199.
Heames, B., Schmitz, J., and Bornberg-Bauer, E., A continuum of evolving de novo genes drives protein-coding novelty in Drosophila, J. Mol. Evol., 2020, vol. 88, no. 4, pp. 382–398.
Heinen, T.J.A.J., Staubach, F., Häming, D., et al., Emergence of a new gene from an intergenic region, Curr. Biol., 2009, vol. 19, no. 18, pp. 1527–1531.
Husnik, F. and McCutcheon, J.P., Functional horizontal gene transfer from bacteria to eukaryotes, Nat. Rev. Microbiol., 2018, vol. 16, no. 2, pp. 67–79.
Ingolia, N.T., Ghaemmaghami, S., Newman, J.R.S., et al., Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling, Science, 2009, vol. 324, no. 5924, pp. 218–223.
Ingolia, N.T., Brar, G.A., Stern-Ginossar, N., et al., Ribosome profiling reveals pervasive translation outside of annotated protein-coding genes, Cell Rep., 2014, vol. 8, no. 5, pp. 1365–1379.
Jacob, F., Evolution and tinkering, Science, 1977, vol. 196, pp. 1161–1166.
Kaessmann, H., Origins, evolution, and phenotypic impact of new genes, Genome Res., 2010, vol. 20, no. 10, pp. 1313–1326.
Kang, M., Ren, M., Li, Y., Fu, Y., et al., Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA, J. Exp. Clin. Cancer Res., 2018, vol. 37.
Kapranov, P., Willingham, A.T., and Gingeras, T.R., Genome-wide transcription and the implications for genomic organization, Nat. Rev. Genet., 2007, vol. 8, no. 6, pp. 413–423.
Kellis, M., Wold, B., Snyder, M.P., et al., Defining functional DNA elements in the human genome, Proc. Natl. Acad. Sci. U. S. A., 2014, vol. 111, no. 17, pp. 6131–6138.
Kim, T.-K., Hemberg, M., Gray, J.M., et al., Widespread transcription at neuronal activity-regulated enhancers, Nature, 2010, vol. 465, pp. 182–187.
Knowles, D.G. and McLysaght, A., Recent de novo origin of human protein-coding genes, Genome Res., 2009, vol. 19, no. 10, pp. 1752–1759.
Kozlov, A.P., Expression of evolutionarily novel genes in tumors, Infect. Agents Cancer, 2016, vol. 11, no. 34.
Kröger, H., Donner, I., and Skiello, G., Influence of a new virostatic compound on the induction of enzymes in rat liver, Arzneimittelforschung, 1975, vol. 25, no. 9, pp. 1426–1429.
Lercher, M.J., Urrutia, A.O., Pavlíček, A., et al., A unification of mosaic structures in the human genome, Hum. Mol. Genet., 2003, vol. 12, no. 19, pp. 2411–2415.
Levine, M.T., Jones, C.D., Kern, A.D., et al., Novel genes derived from noncoding DNA in Drosophila melanogaster are frequently X-linked and exhibit testis-biased expression, Proc. Natl. Acad. Sci. U. S. A., 2006, vol. 103, no. 26, pp. 9935–9939.
Li, L., Foster, C.M., Gan, Q., et al., Identification of the novel protein QQS as a component of the starch metabolic network in Arabidopsis leaves, Plant J., 2009, vol. 58, no. 3, pp. 485–498.
Li, D., Dong, Y., Jiang, Y., et al., A de novo originated gene depresses budding yeast mating pathway and is repressed by the protein encoded by its antisense strand, Cell Res., 2010, vol. 20, no. 4, pp. 408–420.
Li, C.-Y., Zhang, Y., Wang, Z., et al., A human-specific de novo protein-coding gene associated with human brain functions, PLoS Comput. Biol., 2010, vol. 6, no. 3, art. e1000734.
Li, D., Yan, Z., Lu, L., et al., Pleiotropy of the de novo-originated gene MDF1, Sci. Rep., 2014, vol. 4, no. 1, p. 7280.
Li, W., Notani, D., and Rosenfeld, M.G., Enhancers as non-coding RNA transcription units: recent insights and future perspectives, Nat. Rev. Genet., 2016, vol. 17, no. 4, pp. 207–223.
Li, Z.-W., Chen, X., Wu, Q., et al., On the origin of de novo genes in Arabidopsis thaliana populations, Genome Biol. Evol., 2016, vol. 8, no. 7, pp. 2190–2202.
Lin, B., White, J.T., Ferguson, C., et al., PART-1: a novel human prostate-specific, androgen-regulated gene that maps to chromosome 5q12, Cancer Res., 2000, vol. 60, no. 4, pp. 858–863.
Liu, D., Hunt, M., and Tsai, I.J., Inferring synteny between genome assemblies: a systematic evaluation, BMC Bioinformatics, 2018, vol. 19, no. 1, p. 26.
Luis Villanueva-Cañas, J., Ruiz-Orera, J., Agea, M.I., et al., New genes and functional innovation in mammals, Genome Biol. Evol., 2017, vol. 9, no. 7, pp. 1886–1900.
Majic, P. and Payne, J.L., Enhancers facilitate the birth of de novo genes and gene integration into regulatory networks, Mol. Biol. Evol., 2020, vol. 37, no. 4, pp. 1165–1178.
McLysaght, A. and Guerzoni, D., New genes from non-coding sequence: the role of de novo protein-coding genes in eukaryotic evolutionary innovation, Philos. Trans. R. Soc., B, 2015, vol. 370, no. 1678, p. 20140332.
McLysaght, A. and Hurst, L.D., Open questions in the study of de novo genes: what, how and why, Nat. Rev. Genet., 2016, vol. 17, no. 9, pp. 567–578.
Moyers, B.A. and Zhang, J., Phylostratigraphic bias creates spurious patterns of genome evolution, Mol. Biol. Evol., 2015, vol. 32, no. 1, pp. 258–267.
Moyers, B.A. and Zhang, J., Evaluating phylostratigraphic evidence for widespread de novo gene birth in genome evolution, Mol. Biol. Evol., 2016, vol. 33, no. 5, pp. 1245–1256.
Mukherjee, S., Panda, A., and Ghosh, T.C., Elucidating evolutionary features and functional implications of orphan genes in leishmania major, Infect. Genet. Evol., 2015, vol. 32, pp. 330–337.
Muller, H.J., The origination of chromatin deficiencies as minute deletions subject to insertion elsewhere, Genetics, 1935, vol. 17, no. 3, pp. 237–252.
Murphy, D.N. and McLysaght, A., De novo origin of protein-coding genes in murine rodents, PLoS One, 2012, vol. 7, no. 11, art. e48650.
Neme, R. and Tautz, D., Phylogenetic patterns of emergence of new genes support a model of frequent de novo evolution, BMC Genomics, 2013, vol. 14, no. 1, p. 117.
Neme, R. and Tautz, D., Fast turnover of genome transcription across evolutionary time exposes entire non-coding DNA to de novo gene emergence, eLife, 2016, vol. 5, art. e09977.
Nielly-Thibault, L. and Landry, C.R., Differences between the raw material and the products of de novo gene birth can result from mutational biases, Genetics, 2019, vol. 212, no. 4, pp. 1353–1366.
Nishida, H., Detection and characterization of fungal-specific proteins in Saccharomyces cerevisiae, Biosci. Biotechnol. Biochem., 2006, vol. 70, no. 11, pp. 2646–2652.
Ohno, S., Evolution by Gene Duplication, Berlin: Springer-Verlag, 1970.
Ohno, S., Birth of a unique enzyme from an alternative reading frame of the preexisted, internally repetitious coding sequence, Proc. Natl. Acad. Sci. U. S. A., 1984, vol. 81, no. 8, pp. 2421–2425.
Ohno, S., Wolf, U., and Atkin, N.B., Evolution from fish to mammals by gene duplication, Hereditas, 1968, vol. 59, no. 1, pp. 169–187.
Oss, S.B.V. and Carvunis, A.-R., De novo gene birth, PLoS Genet., 2019, vol. 15, no. 5, art. e1008160.
Palmieri, N., Kosiol., C., and Schlötterer, C., The life cycle of Drosophila orphan genes, eLife, 2014, vol. 3, art. e01311.
Papamichos, S.I., Margaritis, D., and Kotsianidis, I., Adaptive evolution coupled with retrotransposon exaptation allowed for the generation of a human-protein-specific coding gene that promotes cancer cell proliferation and metastasis in both haematological malignancies and solid tumours: the extraordinary case of MYEOV gene, Science (Washington, D.C.), 2015, vol. 2015, p. 984706.
Pertea, M., Shumate, A., Pertea, G., et al., Thousands of large-scale RNA sequencing experiments yield a comprehensive new human gene list and reveal extensive transcriptional noise, bioRxiv, 2018, p. 332825.
Potter, S.C., Luciani, A., Eddy, S.R., et al., HMMER web server: 2018 update, Nucleic Acids Res., 2018, vol. 46, pp. W200–W204.
Rancurel, C., Khosravi, M., Dunker, A.K., et al., Overlapping genes produce proteins with unusual sequence properties and offer insight into de novo protein creation, J. Virol., 2009, vol. 83, pp. 10719–10736.
Ranz, J.M., Casals, F., and Ruiz, A., How malleable is the eukaryotic genome? Extreme rate of chromosomal rearrangement in the genus Drosophila, Genome Res., 2001, vol. 11, pp. 230–239.
Reinhardt, J.A., Wanjiru, B.M., Brant, A.T., et al., De novo ORFs in Drosophila are important to organismal fitness and evolved rapidly from previously non-coding sequences, PLoS Genet., 2013, vol. 9, р. e1003860.
Ruiz-Orera, J., Messeguer, X., Subirana, J.A., et al., Long non-coding RNAs as a source of new peptides, eLife, 2014, vol. 3, art. e1003860.
Ruiz-Orera, J., Hernandez-Rodriguez, J., Chiva, C., et al., Origins of de novo genes in human and chimpanzee, PLoS Genet., 2015, vol. 11, art. e1005721.
Samusik, N., Krukovskaya, L., Meln, I., Shilov, E., and Kozlov, A.P., PBOV1 is a human de novo gene with tumor-specific expression that is associated with a positive clinical outcome of cancer, PLoS One, 2013, vol. 8, no. 2, art. e56162.
De Santa, F., Barozzi, I., Mietton, F., et al., A large fraction of extragenic RNA pol II transcription sites overlap enhancers, PLoS Biol., 2010, vol. 8, no. 5, р. e1000384.
Schlötterer, C., Genes from scratch—the evolutionary fate of de novo genes, Trends Genet., 2015, vol. 31, no. 4, pp. 215–219.
Schmidt, E.E., Transcriptional promiscuity in testes, Curr. Biol., 1996, vol. 6, no. 7, pp. 768–769.
Schmitz, J.F., Ullrich, K.K., and Bornberg-Bauer, E., Incipient de novo genes can evolve from frozen accidents that escaped rapid transcript turnover, Nat. Ecol. Evol., 2018, vol. 2, no. 10, pp. 1626–1632.
Schmitz, J.F., Chain, F.J.J., and Bornberg-Bauer, E., Evolution of novel genes in three-spanned stickleback populations, Heredity, 2020, vol. 125, pp. 50–59.
Stark, C., Breitkreutz, B.-J., Chatr-aryamontri, A., et al., The bioGRID interaction database: 2011 update, Nucleic Acids Res., 2011, vol. 39, pp. D698–D704.
Swanson, W.J. and Vacquier, V.D., The rapid evolution of reproductive proteins, Nat. Rev. Genet., 2002, vol. 3, no. 2, pp. 137–144.
Tautz, D. and Domazet-Lošo, T., The evolutionary origin of orphan genes, Nat. Rev. Genet., 2011, vol. 12, no. 10, pp. 692–702.
Toll-Riera, M., Bosch, N., Bellora, N., et al., Origin of primate orphan genes: a comparative genomics approach, Mol. Biol. Evol., 2009, vol. 26, no. 3, pp. 603–612.
Tretyachenko, V., Vymětal, J., Bednárová, L., et al., Random protein sequences can form defined secondary structures and are well-tolerated in vivo, Sci. Rep., 2017, vol. 7, no. 1, p. 15449.
Vakirlis, N., Hebert, A.S., Opulente, D.A., et al., Molecular portrait of de novo genes in yeasts, Mol. Biol. Evol., 2018, vol. 35, no. 3, pp. 631–645.
Vakirlis, N., Carvunis, A.-R., and McLysaght, A., Synteny-based analyses indicate that sequence divergence is not the main source of orphan gene eLife, 2020, vol. 9, art. e53500.
Wang, J., Zhuang, J., Iyer, S., et al., Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors, Genome Res., 2012, vol. 22, no. 9, pp. 1798–1812.
Weisman, C.M., Murray, A.W., and Eddy, S.R., Many, but not all, lineage-specific genes can be explained by homology detection failure, PLoS Biol., 2020, vol. 18, no. 11, art. e3000862.
Werner, M.S., Sieriebriennikov, B., Prabh, N., et al., Young genes have distinct gene structure, epigenetic profiles, and transcriptional regulation, Genome Res., 2018, vol. 28, no. 11, pp. 1675–1687.
Wilson, B.A. and Masel, J., Putatively noncoding transcripts show extensive association with ribosomes, Genome Biol. Evol., 2011, vol. 3, pp. 1245–1252.
Wilson, B.A., Foy, S.G., Neme, R., et al., Young genes are highly disordered as predicted by the preadaptation hypothesis of de novo gene birth, Nat. Ecol. Evol., 2017, vol. 1, no. 6, p. 0146.
Wu, D.-D., Irwin, D.M., and Zhang, Y.-P., De novo origin of human protein-coding genes, PLoS Genet., 2011, vol. 7, no. 11, art. e1002379.
Wu, X. and Sharp, P.A., Divergent transcription: a driving force for new gene origination?, Cell, 2013, vol. 155, no. 5, pp. 990–996.
Xie, C., Bekpen, C., Kunzel, S., et al., Studying the dawn of de novo gene emergence in mice reveals fast integration of new genes into functional networks, bioRxiv, 2019, p. 510214.
Zhang, J.-Y. and Zhou, Q., On the regulatory evolution of new genes throughout their life history, Mol. Biol. Evol., 2019, vol. 36, no. 1, pp. 15–27.
Zhang, Y.E., Landback, P., Vibranovski, M.D., and Long, M., Accelerated recruitment of new brain development genes into the human genome, PLoS Biol., 2011, vol. 9, no. 10, art. e1001179.
Zhang, L., Ren, Y., Yang, T., et al., Rapid evolution of protein diversity by de novo origination in Oryza, Nat. Ecol., 2019, vol. 3, no. 4, pp. 679–690.
Zhang, W., Gao, Y., Long, M., and Shen, B., Origination and evolution of orphan genes and de novo genes in the genome of Caenorhabditis elegans, Sci. China Life Sci., 2019, vol. 62, pp. 579–593.
Zhao, L., Saelao, P., Jones, C.D., et al., Origin and spread of de novo genes in Drosophila melanogaster populations, Science, 2014, vol. 343, no. 6172, pp. 769–772.
Zhou, Q., Zhang, G., Zhang, Y., et al., On the origin of new genes in Drosophila, Genet. Res., 2008, vol. 18, no. 9, pp. 1446–1455.
Zhuang, X., Yang, C., Murphy, K.R., et al., Molecular mechanism and history of non-sense to sense evolution of antifreeze glycoprotein gene in northern gadids, Proc. Natl. Acad. Sci. U. S. A., 2019, vol. 116, no. 10, pp. 4400–4405.
Funding
The work was performed with the financial support of the Russian Foundation for Basic Research (project no. 20-04-00272а) and within the framework of State Assignment of Koltzov Institute of Developmental Biology, Russian Academy of Sciences, 2021, no. 0088-2021-0007 “Molecular Genetic Mechanisms of Regulation of Cell Differentiation and Morphogenesis.”
Author information
Authors and Affiliations
Contributions
Cherezov R.O. analyzed international literature and wrote the main text of the article. Vorontsova Ju.E. participated in the editing of the text of the article and discussion about it. Simonova O.B. initiated the writing of this review and edited the text.
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interests. This paper does not contain any studies involving animals or human participants performed by the authors.
GLOSSARY
Outgroup is a group that is phylogenetically distant from the studied group of sister taxa, originating from a common ancestor, serves as a comparison point for the studied group of taxa.
ncRNA is a noncoding RNA.
Orthologs are homologous genes in different types of organisms, that is, genes that have a common evolutionary origin, a similar structure, and perform a similar function.
ORF is an Open Reading Frame, a section of the gene encoding a protein.
Synteny is the location of genetic loci on the same chromosome, regardless of whether they are linked according to the analysis of linked inheritance.
Synteny blocks are conservative sections of the compared genomes in which the order of arrangement of the studied elements is preserved.
Hidden Markov models are statistical models that are used for recognizing genes, modeling their structure, modeling sequence families, etc.
BLAST (Basic Local Alignment Search Tool) is software that allows you to find areas of similarity between sequences of proteins or nucleotides.
CS-BLAST (Context-Specific BLAST) is software that extends the sensitivity of BLAST to search for similar amino acid sequences.
PSI-BLAST (Position-Specific Iterated BLAST) is a software that extends the capabilities of BLAST to search for similar amino acid sequences, allowing the user to find drastically phylogenetically distant homologous proteins.
k-mers are parts of nucleotide sequences of a certain length (k).
Additional information
Translated by A. Ermakov
Rights and permissions
About this article
Cite this article
Cherezov, R.O., Vorontsova, J.E. & Simonova, O.B. The Phenomenon of Evolutionary “De Novo Generation” of Genes. Russ J Dev Biol 52, 390–400 (2021). https://doi.org/10.1134/S1062360421060035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360421060035