Abstract—
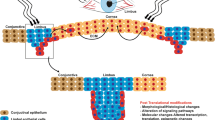
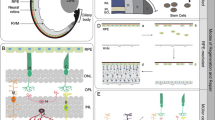
The review examines the role of oxidative stress (OS) in the primary response of the neural eye tissue cells to damage and degenerative processes. The imbalance (homeostasis) of the redox system towards oxidative processes underlies the development of OS. OS can be used as a part of the protective mechanism initiating the processes of healing and regeneration of damaged tissues. Violation of redox homeostasis and OS development triggers an inflammatory response and immune response in the retina. The main reactions to stress (release of ATP, calcium ions, and reactive oxygen species (ROS) into the extracellular space, attraction of exogenous immune cells, activation of endogenous macro- and microglia, apoptosis of neurons) are universal and conservative in all vertebrates. However, with the similarity of some parts of cellular and molecular processes, there are evolutionary fixed functional differences in the regenerative response of retinal cells, and the final result in different vertebrate species is not equivalent. This determines the choice of regeneration strategies: activation of endogenous stem/progenitor cells and/or reprogramming of differentiated cells (retinal pigment epithelium, Müller glia). The detection of key signaling pathways, through which the effect of OS on regenerative responses is realized after damage and pathology of the neural tissues of the vertebral eye, will contribute to the choice of optimal strategies of the cell and/or gene therapy to activate the endogenic regenerative potential of retinal neural tissue in humans.



Similar content being viewed by others
REFERENCES
Abdullayev, I., Kirkham, M., Bjorklund, A.K., et al., A reference transcriptome and inferred proteome for the salamander Notophthalmus viridescens,Exp. Cell Res., 2013, vol. 319, no. 8, pp. 1187–1197.
Ail, D. and Perron, M., Retinal degeneration and regeneration—lessons from fishes and amphibians, Curr. Pathobiol. Rep., 2017, vol. 5, no. 1, pp. 67–78.
Akhtar-Schafer, I., Wang, L., Krohne, T.U., et al., Modulation of three key innate immune pathways for the most common retinal degenerative diseases, EMBO Mol. Med., 2018, vol. 10, no. 10, pp. 1–27. e8259.
Amano, S., Yamagishi, S., Inagaki, Y., et al., Pigment epithelium-derived factor inhibits oxidative stress-induced apoptosis and dysfunction of cultured retinal pericytes, Microvasc. Res., 2005, vol. 69, nos. 1–2, pp. 45–55.
Amram, B., Cohen-Tayar, Y., David, A., et al., The retinal pigmented epithelium - from basic developmental biology research to translational approaches, Int. J. Dev. Biol., 2017, vol. 61, nos. 3–4–5, pp. 225–234.
Anders, H.J. and Schaefer, L., Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis, J. Am. Soc. Nephrol., 2014, vol. 25, no. 7, pp. 1387–1400.
Atienzar-Aroca, S., Flores-Bellver, M., Serrano-Heras, G., et al., Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells, J. Cell Mol. Med., 2016, vol. 20, no. 8, pp. 1457–1466.
Aurora, A.B., Porrello, E.R., Tan, W., Mahmoud, A.I., Hill, J.A., Bassel-Duby, R., Sadek, H.A., and Olson, E.N., Macrophages are required for neonatal heart regeneration, J. Clin. Invest., 2014, vol. 124, no. 3, pp. 1382–1392.
Babaeva, A.G., Reparative processes and immunity, Izv. Akad. Nauk, Ser. Biol., 1999, no. 6, pp. 261–269.
Barbosa-Sabanero, K., Hoffmann, A., Judge, C., et al., Lens and retina regeneration: new perspectives from model organisms, Biochem. J., 2012, vol. 447, no. 3, pp. 321–334.
Beatty, S., Koh, H., Phil, M., et al., The role of oxidative stress in the pathogenesis of age-related macular degeneration, Surv. Ophthalmol., 2000, vol. 45, no. 2, pp. 115–134.
Belin, S., Nawabi, H., Wang, C., et al., Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics, Neuron, 2015, vol. 86, no. 4, pp. 1000–1014.
Bely, A.E. and Nyberg, K.G., Evolution of animal regeneration: re-emergence of a field, Trends Ecol. Evol., 2010, vol. 25, no. 3, pp. 161–170.
Bennis, A., Gorgels, T.G., Ten Brink, J.B., et al., Comparison of mouse and human retinal pigment epithelium gene expression profiles: potential implications for age-related macular degeneration, PLoS One, 2015, vol. 10, no. 10. e0141597.
Bhatia, B., Singhal, S., Jayaram, H., et al., Adult retinal stem cells revisited, Open Ophthalmol. J., 2010, vol. 4, pp. 30–38.
Bi, Y.Y., Feng, D.F., and Pan, D.C., Stem/progenitor cells: a potential source of retina-specific cells for retinal repair, Neurosci. Res., 2009, vol. 65, no. 3, pp. 215–221.
Bindoli, A. and Rigobello, M.P., Principles in redox signaling: from chemistry to functional significance, Antioxid. Redox Signal., 2013, vol. 18, no. 13, pp. 1557–1593.
Blasiak, J., Petrovski, G., Vereb, Z., et al., Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration, Biomed. Res. Int., 2014, vol. 2014, p. 768026.
Boda, E., Nato, G., and Buffo, A., Emerging pharmacological approaches to promote neurogenesis from endogenous glial cells, Biochem. Pharmacol., 2017, vol. 141, pp. 23–41.
Borquez, D.A., Urrutia, P.J., Wilson, C., et al., Dissecting the role of redox signaling in neuronal development, J. Neurochem., 2016, vol. 137, no. 4, pp. 506–517.
Bringmann, A., Iandiev, I., Pannicke, T., et al., Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects, Prog. Retin. Eye Res., 2009, vol. 28, no. 6, pp. 423–451.
Campbell, L.J. and Hyde, D.R., Opportunities for CRISPR/ Cas9 gene editing in retinal regeneration research, Front. Cell Dev. Biol., 2017, vol. 5, art. 99, pp. 1–8.
Casco-Robles, M.M., Islam, M.R., Inami, W., et al., Turning the fate of reprogramming cells from retinal disorder to regeneration by Pax6 in newts, Sci. Rep., 2016, vol. 6, p. 33761.
Chen, H.Y., Kaya, K.D., Dong, L., et al., Three-dimensional retinal organoids from mouse pluripotent stem cells mimic in vivo development with enhanced stratification and rod photoreceptor differentiation, Mol. Vis., 2016, vol. 22, pp. 1077–1094.
Cherenkevich, S.N., Martinovich, G.G., Martinovich, I.V., et al., Redox homeostasis of biological systems: theory and practice, Zh. Gr. Gos. Med. Univ., 2009, no. 2, pp. 9–11.
Chiba, C., The retinal pigment epithelium: an important player of retinal disorders and regeneration, Exp. Eye Res., 2014, vol. 123, pp. 107–114.
Chiba, C. and Mitashov, V.I., Cellular and molecular events in the adult newt retinal regeneration, in The Strategies for Retinal Tissue Repair and Regeneration in Vertebrates: From Fish to Human, Chiba, C., Ed., Kerala, India: Res. Signpost, 2007, pp. 15–33.
Chiba, C., Hoshino, A., Nakamura, K., et al., Visual cycle protein RPE65 persists in new retinal cells during retinal regeneration of adult newt, J. Comp. Neurol., 2006, vol. 495, no. 4, pp. 391–407.
Cho, Y., Shin, J.E., Ewan, E.E., et al., Activating injury-responsive genes with hypoxia enhances axon regeneration through neuronal HIF-1α, Neuron, 2015, vol. 88, no. 4, pp. 720–734.
Chung, J.W., Spot the difference: solving the puzzle of hidden pictures in the lizard genome for identification of regeneration factors, BMB Rep., 2016, vol. 49, no. 5, pp. 249–254.
Conedera, F.M., Pousa, A.M.Q., Mercader, N., et al., Retinal microglia signaling affects Müller cell behavior in the zebrafish following laser injury induction, Glia, 2019, vol. 67, no. 6, pp. 1150–1166.
Cordeiro, J.V. and Jacinto, A., The role of transcription-independent damage signals in the initiation of epithelial wound healing, Nat. Rev. Mol. Cell Biol., 2013, vol. 14, no. 4, pp. 249–262.
Cuenca, N., Fernandez-Sanchez, L., Campello, L., et al., Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases, Prog. Retin. Eye Res., 2014, vol. 43, pp. 17–75.
Cunha-Vaz, J., Bernardes, R., and Lobo, C., Blood-retinal barrier, Eur. J. Ophthalmol., 2011, vol. 21, suppl. 6, pp. 3–9.
Cvekl, A. and Mitton, K.P., Epigenetic regulatory mechanisms in vertebrate eye development and disease, Heredity (Edinb.), 2010, vol. 105, no. 1, pp. 135–151.
Das, A.V., Mallya, K.B., Zhao, X., et al., Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling, Dev. Biol., 2006, vol. 299, no. 1, pp. 283–302.
Delyfer, M.N., Raffelsberger, W., and Mercier, D., Transcriptomic analysis of human retinal detachment reveals both inflammatory response and photoreceptor death, PLoS One, 2011, vol. 6, no. 12. e28791.
Diaz-Coranguez, M., Ramos, C., and Antonetti, D.A., The inner blood-retinal barrier: cellular basis and development, Vision Res., 2017, vol. 139, pp. 123–137.
Dieudonne, S.C., La Heij, E.C., Diederen, R., et al., High TGF-beta2 levels during primary retinal detachment may protect against proliferative vitreoretinopathy, Invest. Ophthalmol. Vis. Sci., 2004, vol. 45, no. 11, pp. 4113–4118.
DiStefano, T., Chen, H.Y., Panebianco, C., et al., Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors, Stem Cell Reports, 2018, vol. 10, no. 1, pp. 300–313.
Dvoriantchikova, G. and Ivanov, D., Tumor necrosis factor-alpha mediates activation of NF-κB and JNK signaling cascades in retinal ganglion cells and astrocytes in opposite ways, Eur. J. Neurosci., 2014, vol. 40, no. 8, pp. 3171–3178.
Dvoriantchikova, G., Seemungal, R.J., and Ivanov, D., The epigenetic basis for the impaired ability of adult murine retinal pigment epithelium cells to regenerate retinal tissue, Sci. Rep., 2019, vol. 9, no. 1, pp. 1–13.
Elewa, A., Wang, H., and Talavera-Lopez, C., Reading and editing the Pleurodeles waltl genome reveals novel features of tetrapod regeneration, Nat. Commun., 2017, vol. 8, no. 1, pp. 1–9.
Emanuele, S., D’Anneo, A., Calvaruso, G., et al., The double-edged sword profile of redox signaling: oxidative events as molecular switches in the balance between cell physiology and cancer, Chem. Res. Toxicol., 2018, vol. 31, no. 4, pp. 201–210.
Erler, P. and Monaghan, J.R., The link between injury-induced stress and regenerative phenomena: a cellular and genetic synopsis, Biochim. Biophys. Acta, 2015, vol. 1849, no. 4, pp. 454–461.
Ferretti, P., Is there a relationship between adult neurogenesis and neuron generation following injury across evolution?, Eur. J. Neurosci., 2011, vol. 34, no. 6, pp. 951–962.
Fischer, R. and Maier, O., Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF, Oxid. Med. Cell. Longev., 2015, vol. 2015, article ID 610813, pp. 1–18.
Fischer, A.J. and Reh, T.A., Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina, Dev. Biol., 2002, vol. 251, no. 2, pp. 367–379.
Fisher, S.K., Lewis, G.P., Linberg, K.A., et al., Cellular remodeling in mammalian retina induced by retinal detachment, in Webvision: The Organization of the Retina and Visual System [Internet], Kolb, H., Fernandez, E., and Nelson, R., Eds., University of Utah Health Sciences Center, Salt Lake City, UT, USA, 2007, pp. 1–51.
Fuhrmann, S., Eye morphogenesis and patterning of the optic vesicle, Curr. Top. Dev. Biol., 2010, vol. 93, pp. 61–84.
Fukui, M. and Zhu, B.T., Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells, Free Radic. Biol. Med., 2010, vol. 48, no. 6, pp. 821–830.
Galliot, B. and Chera, S., The hydra model: disclosing an apoptosis-driven generator of Wnt-based regeneration, Trends Cell Biol., 2010, vol. 20, no. 9, pp. 514–523.
Galliot, B. and Ghila, L., Cell plasticity in homeostasis and regeneration, Mol. Reprod. Dev., 2010, vol. 77, no. 10, pp. 837–855.
Galliot, B., Crescenzi, M., Jacinto, A., et al., Trends in tissue repair and regeneration, Development, 2017, vol. 144, no. 3, pp. 357–364.
Galluzzi, L., Bravo-San Pedro, J.M., Kepp, O., et al., Regulated cell death and adaptive stress responses, Cell. Mol. Life Sci., 2016, vol. 73, nos. 11–12, pp. 2405–2410.
Gasparini, S.J., Llonch, S., Borsch, O., et al., Transplantation of photoreceptors into the degenerative retina: current state and future perspectives, Prog. Retin. Eye Res., 2019, vol. 69, pp. 1–37.
Geller, S.F., Lewis, G.P., and Fisher, S.K., Fgfr1, signaling, and ap-1 expression after retinal detachment: reactive Müller and RPE cells, Invest. Ophthalmol. Vis. Sci., 2001, vol. 42, pp. 1363–1369.
Genini, S., Beltran, W.A., Stein, V.M., et al., Isolation and ex vivo characterization of the immunophenotype and function of microglia/macrophage populations in normal dog retina, Adv. Exp. Med. Biol., 2014, vol. 801, pp. 339–345.
Grigoryan, E.N., Shared triggering mechanisms of retinal regeneration in lower vertebrates and retinal rescue in higher ones, in Tissue Regeneration—From Basic Biology to Clinical Application, Davies, J., Ed., Croatia: In Tech, 2012, pp. 145–164.
Grigoryan, E.N. and Markitantova, Y.V., Cellular and molecular preconditions for retinal pigment epithelium (RPE) natural reprogramming during retinal regeneration in Urodela, Biomedicines, 2016, vol. 4, no. 4, pii: E28, pp. 1–18.
Hameed, L.S., Berg, D.A., Belnoue, L., et al., Environmental changes in oxygen tension reveal ROS-dependent neurogenesis and regeneration in the adult newt brain, Elife, 2015, vol. 20, no. 4, pii: e08422, pp. 1–16.
Hamon, A., Roger, J.E., Yang, X.J., et al., Müller glial cell-dependent regeneration of the neural retina: an overview across vertebrate model systems, Dev. Dyn., 2016, vol. 245, no. 7, pp. 727–738.
Haynes, T., Luz-Madrigal, A., Reis, E.S., et al., Complement anaphylatoxin C3a is a potent inducer of embryonic chick retina regeneration, Nat. Commun., 2013, vol. 4, p. 2312.
Hoon, M., Okawa, H., Della Santina, L., et al., Functional architecture of the retina: development and disease, Prog. Retin. Eye Res., 2014, vol. 42, pp. 44–84.
de Hoz, R., Rojas, B., Ramírez, A.I., et al., Retinal macroglial responses in health and disease, Biomed. Res. Int., 2016, vol. 2016, article ID 2954721, pp. 1–13.
Hunter, J.J., Morgan, J.I., Merigan, W.H., et al., The susceptibility of the retina to photochemical damage from visible light, Prog. Retin. Eye Res., 2012, vol. 31, no. 1, pp. 28–42.
Inami, W., Islam, M.R., Nakamura, K., et al., Expression of two classes of pax6 transcripts in reprogramming retinal pigment epithelium cells of the adult newt, Zoolog. Sci., 2016, vol. 33, no. 1, pp. 21–30.
Iqbal, J., Zhang, K., Jin, N., et al., Alzheimer’s disease is responsible for progressive age-dependent differential expression of various protein cascades in retina of mice, ACS Chem. Neurosci., 2019. https://doi.org/10.1021/acschemneuro.8b00710
Isenmann, S., Kretz, A., and Cellerino, A., Molecular determinants of retinal ganglion cell development, survival, and regeneration, Prog. Retin. Eye Res., 2003, vol. 22, no. 4, pp. 483–543.
Islam, M.R., Nakamura, K., Casco-Robles, M.M., et al., The newt reprograms mature RPE cells into a unique multipotent state for retinal regeneration, Sci. Rep., 2014, vol. 4, pp. 1–8.
Jacobs, A.T. and Marnett, L.J., Systems analysis of protein modification and cellular responses induced by electrophile stress, Acc. Chem. Res., 2010, vol. 43, no. 5, pp. 673–683.
Jarrett, S.G. and Boulton, M.E., Antioxidant up-regulation and increased nuclear DNA protection play key roles in adaptation to oxidative stress in epithelial cells, Free Radic. Biol. Med., 2005, vol. 38, no. 10, pp. 1382–1391.
Jorstad, N.L., Wilken, M.S., Grimes, W.N., et al., Stimulation of functional neuronal regeneration from Müller glia in adult mice, Nature, 2017, vol. 548, no. 7665, pp. 103–107.
Kaneko, Y., Matsumoto, G., Hanyu, Y., et al., The occurrence of apoptosis during retinal regeneration in adult newts, Brain Res. Dev. Brain Res., 1999, vol. 117, no. 2, pp. 225–228.
Kang, M.K., Lee, E.J., Kim, Y.H., et al., Chrysin ameliorates malfunction of retinoid visual cycle through blocking activation of AGE-RAGE-ER stress in glucose-stimulated retinal pigment epithelial cells and diabetic eyes, Nutrients, 2018, vol. 10, no. 8. pii: E1046.
Katsman, D., Stackpole, E.J., Domin, D.R., et al., Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina, PLoS One, 2012, vol. 7, no. 11. e50417.
Keeling, E., Lotery, A.J., Tumbarello, D.A., et al., Impaired cargo clearance in the retinal pigment epithelium (RPE) underlies irreversible blinding diseases, Cells, 2018, vol. 7, no. 2. pii: E16.
Kimelberg, H.K., Functions of mature mammalian astrocytes: a current view, Neuroscientist, 2010, vol. 16, no. 1, pp. 79–106.
Kirchhof, B. and Sorgente, N., Pathogenesis of proliferative vitreoretinopathy. Modulation of retinal pigment epithelial cell functions by vitreous and macrophages, Dev. Ophthalmol., 1989, vol. 16, pp. 1–53.
Kolb, H., Outer plexiform layer, in Webvision: The Organization of the Retina and Visual System [Internet], Kolb, H., Fernandez, E., and Nelson, R., Eds., University of Utah Health Sciences Center, Salt Lake City, UT, USA, 2007a.
Kolb, H., Inner plexiform layer, in Webvision: The Organization of the Retina and Visual System [Internet], Kolb, H., Fernandez, E., and Nelson, R., Eds., University of Utah Health Sciences Center, Salt Lake City, UT, USA, 2007b.
Kolb, H., Simple anatomy of the retina, in Webvision: The Organization of the Retina and Visual System [Internet], Kolb, H., Fernandez, E., and Nelson, R., Eds., University of Utah Health Sciences Center, Salt Lake City, UT, USA, 2012.
Kortuem, K., Geiger, L.K., and Levin, L.A., Differential susceptibility of retinal ganglion cells to reactive oxygen species, Invest. Ophthalmol. Vis. Sci., 2000, vol. 41, no. 10, pp. 3176–3182.
Kostas, M., Lampart, A., Bober, J., et al., Translocation of exogenous FGF1 and FGF2 protects the cell against apoptosis independently of receptor activation, J. Mol. Biol., 2018, vol. 430, no. 21, pp. 4087–4101.
Kozakowska, M., Pietraszek-Gremplewicz, K., Jozkowicz, A., et al., The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes, J. Muscle Res. Cell Motil., 2015, vol. 36, no. 6, pp. 377–393.
Kumaramanickavel, G., Age-related macular degeneration: genetics and biology, Asia Pac. J. Ophthalmol. (Phila), 2016, vol. 5, no. 4, pp. 229–235.
Lamb, T.D., Evolution of phototransduction, vertebrate photoreceptors and retina, Prog. Retin. Eye Res., 2013, vol. 36, pp. 52–119.
Langhe, R., Chesneau, A., Colozza, G., et al., Müller glial cell reactivation in Xenopus models of retinal degeneration, Glia, 2017, vol. 65, no. 8, pp. 1333–1349.
Leaver, S.G., Cui, Q., Plant, G.W., et al., AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells, Gene Ther., 2006, vol. 13, no. 18, pp. 1328–1341.
Lenkowski, J.R. and Raymond, P.A., Müller glia: stem cells for generation and regeneration of retinal neurons in teleost fish, Prog. Retin. Eye Res., 2014, vol. 40, pp. 94–123.
Leveillard, T. and Sahel, J.A., Metabolic and redox signaling in the retina, Cell Mol. Life Sci., 2017, vol. 74, no. 20, pp. 3649–3665.
Li, F., Huang, Q., Chen, J., et al., Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration, Sci. Signal., 2010, vol. 3, no. 110. ra13, pp. 1–20.
Llonch, S., Carido, M., and Ader, M., Organoid technology for retinal repair, Dev. Biol., 2018, vol. 433, no. 2, pp. 132–143.
Logan, A., Ahmed, Z., Baird, A., et al., Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury, Brain, 2006, vol. 129, pt 2, pp. 490–502.
Love, N.R., Chen, Y., Ishibashi, S., et al., Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration, Nat. Cell Biol., 2013, vol. 15, no. 2, pp. 222–228.
Lu, Q., Cui, Q., Yip, H.K., et al., c-Jun expression in surviving and regenerating retinal ganglion cells: effects of intravitreal neurotrophic supply, Invest. Ophthalmol. Vis. Sci., 2003, vol. 44, no. 12, pp. 5342–5348.
Lushchak, V.I., Adaptive response to oxidative stress: bacteria, fungi, plants and animals, Comp. Biochem. Physiol. C. Toxicol. Pharmacol., 2011, vol. 153, no. 2, pp. 175–190.
Ma, W., Zhang, Y., Gao, C., et al., Monocyte infiltration and proliferation reestablish myeloid cell homeostasis in the mouse retina following retinal pigment epithelial cell injury, Sci. Rep., 2017, vol. 7, no. 1, 8433, pp. 1–18.
Markitantova, Yu.V., Avdonin, P.P., and Grigoryan, E.N., Specific activity of fibroblast growth factor 2 and nucleostemin in forming vertebrate retina, in Nauchnaya konferentsiya s mezhdunarodnym uchastiem “Aktual’nye voprosy morfogeneza v norme i patologii”, 6–7 aprelya 2016, Moskva. Sbornik nauchnykh trudov” (Sci. Conf. with Int. Particip. “Topical Issues of Morphogenesis in Health and Disease,” April 6–7, 2016, Moscow. Collected Scientific Papers), Moscow: Gruppa MDV, 2016, pp. 107–108.
Masutomi, K., Chen, C., Nakatani, K., et al., All-trans retinal mediates light-induced oxidation in single living rod photoreceptors, Photochem. Photobiol., 2012, vol. 88, no. 6, pp. 1356–1361.
Mazzolini, M., Facchetti, G., Andolfi, L., et al., The phototransduction machinery in the rod outer segment has a strong efficacy gradient, Proc. Natl. Acad. Sci. U. S. A., 2015, vol. 112, no. 20, pp. E2715–24.
Meda, F., Rampon, C., Dupont, E., et al., Nerves, H2O2 and Shh: three players in the game of regeneration, Semin. Cell Dev. Biol., 2018, vol. 80, pp. 65–73.
Mishra, S., Maurya, Sh.K., Srivastava, K., Shukla, S., and Mishra, R., Pax6 influences expression patterns of genes involved in neurodegeneration, Ann. Neurosci., 2015, vol. 22, no. 4, pp. 226–231.https://doi.org/10.5214/ans.0972.7531.220407
Mitashov, V.I., Mechanisms of retina regeneration in urodeles, Int. J. Dev. Biol., 1996, vol. 40, no. 4, pp. 833–844.
Mitashov, V.I., Retinal regeneration in amphibians, Int. J. Dev. Biol., 1997, vol. 41, no. 6, pp. 893–905.
Mitchell, C.H., Lu, W., Hu, H., et al., The P2X(7) receptor in retinal ganglion cells: a neuronal model of pressure-induced damage and protection by a shifting purinergic balance, Purinergic Signal., 2008, vol. 4, no. 4, pp. 313–321.
Mitra, S., Sharma, P., Kaur, S., et al., Dual regulation of lin28a by Myc is necessary during zebrafish retina regeneration, J. Cell Biol., 2019, vol. 218, no. 2, pp. 489–507.
Moldogazieva, N.T., Mokhosoev, I.M., Feldman, N.B., et al., ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications, Free Radic. Res., 2018, vol. 52, no. 5, pp. 507–543.
Murawala, P., Tanaka, E.M., and Currie, J.D., Regeneration: the ultimate example of wound healing, Semin. Cell Dev. Biol., 2012, vol. 23, no. 9, pp. 954–962.
Nabeshima, A., Nishibayashi, C., Ueda, Y., et al., Loss of cell-extracellular matrix interaction triggers retinal regeneration accompanied by Rax and Pax6 activation, Genesis, 2013, vol. 51, no. 6, pp. 410–419.
Nagashima, M., Fujikawa, C., Mawatari, K., et al., HSP70, the earliest-induced gene in the zebrafish retina during optic nerve regeneration: its role in cell survival, Neurochem. Int., 2011, vol. 58, no. 8, pp. 888–895.
Nakamura, K. and Chiba, C., Evidence for Notch signaling involvement in retinal regeneration of adult newt, Brain Res., 2007, vol. 1136, no. 1, pp. 28–42.
Nakamura, K., Islam, M.R., Takayanagi, M., et al., A transcriptome for the study of early processes of retinal regeneration in the adult newt, Cynops pyrrhogaster, PLoS One, 2014, vol. 9, no. 10, e109831, pp. 1–12.
Nakanishi, T., Shimazawa, M., Sugitani, S., et al., Role of endoplasmic reticulum stress in light-induced photoreceptor degeneration in mice, J. Neurochem., 2013, vol. 125, no. 1, pp. 111–124.
Nakanishi-Ueda, T., Majima, H.J., Watanabe, K., et al., Blue LED light exposure develops intracellular reactive oxygen species, lipid peroxidation, and subsequent cellular injuries in cultured bovine retinal pigment epithelial cells, Free Radic. Res., 2013, vol. 47, no. 10, pp. 774–780.
Nakazawa, T., Matsubara, A., Noda, K., et al., Characterization of cytokine responses to retinal detachment in rats, Mol. Vis., 2006, vol. 12, pp. 867–878.
Neves, J., Zhu, J., Sousa-Victor, P., et al., Immune modulation by MANF promotes tissue repair and regenerative success in the retina, Science, 2016, vol. 353, no. 6294. aaf3646.
Newman, E.A., Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters, Philos. Trans. R. Soc. Lond. Ser. Biol., 2015, vol. 370, no. 1672.
Noro, T., Namekata, K., Kimura, A., et al., Spermidine promotes retinal ganglion cell survival and optic nerve regeneration in adult mice following optic nerve injury, Cell Death Dis., 2015, vol. 6. e1720.
Nowoshilow, S., Schloissnig, S., Fei, J.F., et al., The axolotl genome and the evolution of key tissue formation regulators, Nature, 2018, vol. 554, no. 7690, pp. 50–55.
Osakada, F., Ooto, S., Akagi, T., et al., Wnt signaling promotes regeneration in the retina of adult mammals, J. Neurosci., 2007, vol. 27, no. 15, pp. 4210–4219.
Perez-Torres, I., Guarner-Lans, V., and Rubio-Ruiz, M.E., Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents, Int. J. Mol. Sci., 2017, vol. 18, no. 10. pii: E2098, pp. 1–26.
Powell, C., Grant, A.R., Cornblath, E., et al., Analysis of DNA methylation reveals a partial reprogramming of the Müller glia genome during retina regeneration, Proc. Natl. Acad. Sci. U. S. A., 2013, vol. 110, no. 49, pp. 19814–19819.
Rao, M.B., Didiano, D., and Patton, J.G., Neurotransmitter-regulated regeneration in the zebrafish retina, Stem Cell Rep., 2017, vol. 8, no. 4, pp. 831–842.
Ray, P.D., Huang, B.W., and Tsuji, Y., Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling, Cell Signal., 2012, vol. 24, no. 5, pp. 981–990.
Reczek, C.R. and Chandel, N.S., ROS-dependent signal transduction, Curr. Opin. Cell Biol., 2015, vol. 33, pp. 8–13.
Reichenbach, A. and Bringmann, A., New functions of Müller cells, Glia, 2013, vol. 61, no. 5, pp. 651–678.
Reichenbach, A. and Bringmann, A., Role of purines in Müller glia, J. Ocul. Pharmacol. Ther., 2016, vol. 32, no. 8, pp. 518–533.
Roehlecke, C., Schumann, U., Ader, M., et al., Stress reaction in outer segments of photoreceptors after blue light irradiation, PLoS One, 2013, vol. 8, no. 9, e71570, pp. 1–12.
Roy, S. and Levesque, M., Limb regeneration in axolotl: is it superhealing?, Sci. World J., 2006, vol. 6, suppl. 1, pp. 12–25.
Salero, E., Blenkinsop, T.A., Corneo, B., et al., Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives, Cell Stem Cell, 2012, vol. 10, no. 1, pp. 88–95.
Sanes, J.R. and Masland, R.H., The types of retinal ganglion cells: current status and implications for neuronal classification, Annu. Rev. Neurosci., 2015, vol. 38, pp. 221–246.
Sato, K., Saigusa, D., Saito, R., et al., Metabolomic changes in the mouse retina after optic nerve injury, Sci. Rep., 2018, vol. 8, no. 1, p. 11930.
Schlingemann, R.O., Role of growth factors and the wound healing response in age-related macular degeneration, Graefes Arch. Clin. Exp. Ophthalmol., 2004, vol. 242, no. 1, pp. 91–101.
Seagle, B.L., Rezai, K.A., Gasyna, E.M., et al., Time-resolved detection of melanin free radicals quenching reactive oxygen species, J. Am. Chem. Soc., 2005, vol. 127, no. 32, pp. 11220–11221.
Selvam, S., Kumar, T., and Fruttiger, M., Retinal vasculature development in health and disease, Prog. Retin. Eye Res., 2018, vol. 63, pp. 1–19.
Sharma, T.P., McDowell, C.M., Liu, Y., et al., Optic nerve crush induces spatial and temporal gene expression patterns in retina and optic nerve of BALB/cJ mice, Mol. Neurodegener., 2014, vol. 9, no. 14, pp. 1–19.
Sies, H., Berndt, C., and Jones, D.P., Oxidative stress, Annu. Rev. Biochem., 2017, vol. 86, pp. 715–748.
Sifuentes, C.J., Kim, J.W., Swaroop, A., et al., Rapid, dynamic activation of Müller glial stem cell responses in zebrafish, Invest. Ophthalmol. Vis. Sci., 2016, vol. 57, no. 13, pp. 5148–5160.
Simo, R. and Hernandez, C., European consortium for the early treatment of diabetic retinopathy (EUROCONDOR) neurodegeneration in the diabetic eye: new insights and therapeutic perspectives, Trends Endocrinol. Metabol., 2014, vol. 25, no. 1, pp. 23–33.
Simon, M.V., Agnolazza, D.L., German, O.L., et al., Synthesis of docosahexaenoic acid from eicosapentaenoic acid in retina neurons protects photoreceptors from oxidative stress, J. Neurochem., 2016, vol. 136, no. 5, pp. 931–946.
Singh, R., Cuzzani, O., Binette, F., et al., Pluripotent stem cells for retinal tissue engineering: current status and future prospects, Stem Cell Rev., 2018, vol. 14, no. 4, pp. 463–483.
Slack, J.M., Beck, C.W., Gargioli, C., et al., Cellular and molecular mechanisms of regeneration in Xenopus, Philos. Trans. R Soc. Lond.,Ser. Biol., 2004, vol. 359, no. 1445, pp. 745–751.
Solberg, R., Escobar, J., Arduini, A., et al., Metabolomic analysis of the effect of postnatal hypoxia on the retina in a newly born piglet model, PLoS One, 2013, vol. 8, no. 6. e66540.
Sousounis, K., Looso, M., Maki, N., et al., Transcriptome analysis of newt lens regeneration reveals distinct gradients in gene expression patterns, PLoS One, 2013, no. 4, p. 8. e61445.
Sousounis, K., Bhavsar, R., Looso, M., et al., Molecular signatures that correlate with induction of lens regeneration in newts: lessons from proteomic analysis, Hum. Genomics, 2014, vol. 8, 22, pp. 1–16.
Stevenson, L., Matesanz, N., Colhoun, L., et al., Reduced nitro-oxidative stress and neural cell death suggests a protective role for microglial cells in TNFalpha–/– mice in ischemic retinopathy, Invest. Ophthalmol. Vis. Sci., 2010, vol. 51, no. 6, pp. 3291–3299.
Strauss, O., The retinal pigment epithelium in visual function, Physiol. Rev., 2005, vol. 85, no. 3, pp. 845–881.
Subirada, P.V., Paz, M.C., Ridano, M.E., et al., A journey into the retina: Müller glia commanding survival and death, Eur. J. Neurosci., 2018, vol. 47, no. 12, pp. 1429–1443.
Sun, M., Finnemann, S.C., Febbraio, M., et al., Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions, J. Biol. Chem., 2006, vol. 281, no. 7, pp. 4222–4230.
Suzuki, N. and Mittler, R., Reactive oxygen species-dependent wound responses in animals and plants, Free Radic. Biol. Med., 2012, vol. 53, no. 12, pp. 2269–2276.
Tate, D.J., Jr., Miceli, M.V., and Newsome, D.A., Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells, Invest. Ophthalmol. Vis. Sci., 1995, vol. 36, no. 7, pp. 1271–1279.
Todd, L., Suarez, L., Quinn, C., et al., Retinoic acid-signaling regulates the proliferative and neurogenic capacity of Müller glia-derived progenitor cells in the avian retina, Stem Cells, 2018, vol. 36, no. 3, pp. 392–405.
Tokarz, P., Kaarniranta, K., and Blasiak, J., Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD), Biogerontology, 2013, vol. 14, no. 5, pp. 461–482.
Tonges, L., Ostendorf, T., Lamballe, F., et al., Hepatocyte growth factor protects retinal ganglion cells by increasing neuronal survival and axonal regeneration in vitro and in vivo, J. Neurochem., 2011, vol. 117, no. 5, pp. 892–903.
Trachootham, D., Lu, W., Ogasawara, M.A., et al., Redox regulation of cell survival, Antioxid. Redox Signal., 2008, vol. 10, no. 8, pp. 1343–1374.
Trost, A., Bruckner, D., Rivera, F.J., et al., Pericytes in the retina, Adv. Exp. Med. Biol., 2019, vol. 1122, pp. 1–26.
Tsukamoto, Y., Morphological survey from neurons to circuits of the mouse retina, Methods Mol. Biol., 2018, vol. 1753, pp. 3–25.
Ueta, T., Inoue, T., Furukawa, T., et al., Glutathione peroxidase 4 is required for maturation of photoreceptor cells, J. Biol. Chem., 2012, vol. 287, no. 10, pp. 7675–7682.
Usui, S., Oveson, B.C., Iwase, T., et al., Overexpression of sod in retina: need for increase in H2O2-detoxifying enzyme in same cellular compartment, Free Radic. Biol. Med., 2011, vol. 51, no. 7, pp. 1347–1354.
Vigneswara, V., Esmaeili, M., Deer, L., et al., Eye drop delivery of pigment epithelium-derived factor-34 promotes retinal ganglion cell neuroprotection and axon regeneration, Mol. Cell. Neurosci., 2015, vol. 68, pp. 212–221.
van der Vliet, A. and Janssen-Heininger, Y.M., Hydrogen peroxide as a damage signal in tissue injury and inflammation: murderer, mediator, or messenger?, J. Cell. Biochem., 2014, vol. 115, no. 3, pp. 427–435.
Vriz, S., Reiter, S., and Galliot, B., Cell death: a program to regenerate, Curr. Top. Dev. Biol., 2014, vol. 108, pp. 121–151.
Wan, J. and Goldman, D., Retina regeneration in zebrafish, Curr. Opin. Genet. Dev., 2016, vol. 40, pp. 41–47.
Wan, J., Ramachandran, R., and Goldman, D., HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration, Dev. Cell, 2012, vol. 22, no. 2, pp. 334–347.
Wang, Z., Dillon, J., and Gaillard, E.R., Antioxidant properties of melanin in retinal pigment epithelial cells, Photochem. Photobiol., 2006, vol. 82, no. 2, pp. 474–479.
Wang, S., Chu, C.H., Stewart, T., et al., α-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation, Proc. Natl. Acad. Sci. U. S. A., 2015, vol. 112, no. 15, pp. E1926–E1935.
Webster, M.K., Barnett, B.J., Stanchfield, M.L., et al., Stimulation of retinal pigment epithelium with an α7 nAChR agonist leads to Müller glia dependent neurogenesis in the adult mammalian retina, Invest. Ophthalmol. Vis. Sci., 2019, vol. 60, no. 2, pp. 570–579.
Werdich, X.Q., Place, E.M., and Pierce, E.A., Systemic diseases associated with retinal dystrophies, Semin. Ophthalmol., 2014, vol. 29, nos. 5–6, pp. 319–328.
Williams, C.J. and Dexter, D.T., Neuroprotective and symptomatic effects of targeting group III mGlu receptors in neurodegenerative disease, J. Neurochem., 2014, vol. 129, no. 1, pp. 4–20.
Winterbourn, C.C. and Kettle, A.J., Redox reactions and microbial killing in the neutrophil phagosome, Antioxid. Redox Signal., 2013, vol. 18, no. 6, pp. 642–660.
Yu, A.L., Fuchshofer, R., Kook, D., et al., Subtoxic oxidative stress induces senescence in retinal pigment epithelial cells via TGF-beta release, Invest. Ophthalmol. Vis. Sci., 2009, vol. 50, no. 2, pp. 926–935.
Zacks, D.N., Han, Y., Zeng, Y., et al., Activation of signaling pathways and stress-response genes in an experimental model of retinal detachment, Invest. Ophthalmol. Vis. Sci., 2006, vol. 47, no. 4, pp. 1691–1695.
Zacks, D.N., Gene transcription profile of the detached retina (An AOS Thesis), Trans. Am. Ophthalmol. Soc., 2009, vol. 107, pp. 343–382.
Zareba, M., Sarna, T., and Szewczyk, G., Photobleaching of melanosomes from retinal pigment epithelium: II. Effects on the response of living cells to photic stress, Photochem. Photobiol., 2007, vol. 83, no. 4, pp. 925–930.
Zhang, X., Zhang, M., Laties, A.M., et al., Balance of purines may determine life or death of retinal ganglion cells as A3 adenosine receptors prevent loss following P2X7 receptor stimulation, J. Neurochem., 2006, vol. 98, no. 2, pp. 566–575.
Zhang, S.X., Sanders, E., Fliesler, S.J., et al., Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration, Exp. Eye. Res., 2014, vol. 125, pp. 30–40.
Zhao, X.F., Wan, J., Powell, C., et al., Leptin and IL-6 family cytokines synergize to stimulate Müller glia reprogramming and retina regeneration, Cell Rep., 2014, vol. 9, no. 1, pp. 272–284.
Zhao, L., Zabel, M.K., Wang, X., et al., Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration, EMBO Mol. Med., 2015, vol. 7, no. 9, pp. 1179–1197.
Zhou, G., Meng, S., Li, Y., et al., Optimal ROS signaling is critical for nuclear reprogramming, Cell Rep., 2016, vol. 15, no. 5, pp. 919–925.
Funding
This work was supported by the program of the Presidium of the Russian Academy of Sciences “Biodiversity of Natural Systems” within the framework of IDB RUS Government Basic Research Program no. 0108-2018-0005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Markitantova, Y.V., Simirskii, V.N. Role of the Redox System in Initiation of a Regenerative Response of Neural Eye Tissues in Vertebrates. Russ J Dev Biol 51, 16–30 (2020). https://doi.org/10.1134/S106236042001004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106236042001004X