Abstract
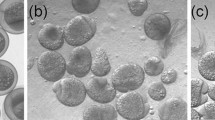
An attempt was undertaken to develop a model system based on artificial cell cycle synchronization by means of reversible mitosis blocking in zebrafish embryos for studying the role of cell cycle synchrony in embryogenesis. Dechorionized and intact embryos at the stages of 512-cell blastula and 75% epiboly were treated with nocodazole and then washed within several times of exposure. When working on dechorionized embryos, we succeeded to obtain complete block mitosis in the presence of low nocodazole concentrations: 0.5–1.0 μg/ml. Block of mitosis was relieved in all experimental series within a certain time after the beginning of washing. This inertia depended on both nocodazole concentration and duration of treatment. The nocodazole elimination was significantly accelerated only after five (or more) changes of washing medium containing DMSO. As a result, the conditions were established for obtaining a parasynchronous cell population in the zebrafish gastrulas with a peak of mitosis up to 17.2%.
Similar content being viewed by others
References
Adams, R., Metody kul’tury kletok dlya biokhimikov (Methods of Cell Culture for Biochemists), Moscow: Mir, 1983.
El Ali, M.Sh., Effect of Colchamine on Segmentation of Paraxial Mesoderm in Chick Embryo, Cand. Sci. (Biol.) Dissertation, St. Petersburg: SPbGU, 1993.
Atés, Y. and Sentein, P., Action of Nocodazole on Segmentation Mitoses of Triturus helveticus Raz. Electron Microscopy, Biol. Cells, 1981, vol. 40, pp. 175–180.
Baumann, M. and Sander, K., Bipartite Axiation Follows Incomplete Epiboly in Zebrafish Embryos Treated with Chemical Teratogens, J. Exp. Zool., 1984, vol. 230, pp. 363–376.
Cleaver, J.E., Thymidine Metabolism and Cell Kinetics, Amsterdam: North Holland Publ. Co, 1967.
De Brabander, M.J., Van de Veire, R.M.L., Aerts, F.E.M., et al., The Effects of Methyl [5-(2-Thienylcarbonyl)-1H-Benzimidzol-2-yl]Carbamate, (R 17934; NSC 238159), a New Synthetic Antitumoral Drug Interfering with Microtubules, on Mammalian Cells Culture in Vitro, Cancer Res., 1976, vol. 36, pp. 905–916.
Dondua, A.K. and Fedorova, Zh.E., Mitotic Cycles during Formation of Neural Tube and Somites in Chick Embryo under Culture Conditions, Issledovaniya kletochnykh tsiklov i metabolizma nukleinovykh kislot pri differentsiatsii kletok (Studies of Cell Cycles and Nucleic Acid Metabolism during Cell Differentiation), Moscow: Nauka, 1964, pp. 83–89.
Dondua, A.K., Cell Cycles in Early Animal Development, Kletochnoe razmnozhenie i protsessy differentsirovki (Cell Proliferation and Differentiation), Moscow: Nauka, 1983, pp. 6–75.
Emanuelsson, H., Mitotic Activity in Chick Embryos at the Primitive Streak Stage, Acta Physiol. Scand., 1961, vol. 52, pp. 211–233.
Foe, V.E., Mitotic Doamins Reveal Early Commitment of Cells of Drosophila Embryos, Development, 1989, vol. 107, pp. 1–22.
Kane, D.A. and Kimmel, C.B., The Zebrafish Midblastula Transition, Devel. Biol., 1993, vol. 119, pp. 447–456.
Kato, Y. and Tsunoda, Y., Synchronous Division of Mouse Two-Cell Embryos with Nocodazole in Vitro, J. Reprod. Fertil., 1992, vol. 95, pp. 39–43.
Kimmel, C.B., Ballard, W.W., and Kimmel, S.R., Stages of Embryonic Development of the Zebrafish, Devel. Dynamics, 1995, vol. 203, pp. 253–310.
Mareel, M., Bellairs, R., De Bruyne, G., and Van Peteghem, M.C., Effect of Microtubule Inhibitors on the Expansion of Hypoblast and Margin of Overgrowth of Chick Blastoderm S, J. Embryol. Exp. Morphol., 1984, vol. 81, pp. 273–286.
Neyfakh, A.A. and Rott, N.N., Synchronization of Cell Division in Early Embryos of the Loach Misgurnus fossilis L. by Elevated Temperature, Dokl. Akad. Nauk. SSSR, 1958, vol. 119, no. 2, pp. 432–434.
Neyfakh, A.A., Application of the Method of Radiation Inactivation of the Nuclei for Studying Their Function in Early Development of Fish, Zh. Obshch. Biol., 1959, vol. 20, no. 3, pp. 202–213.
Neyfakh, A.A., Comparative Radiation Studies of Nuclear Morphogenetic Function in Animal Development, Zh. Obshch. Biol., 1961, vol. 22, no. 1, pp. 42–57.
Newport, J. and Kirschner, M., A Major Developmental Transition in Early Xenopus Embryos. 1. Characterization and Timing of Cellular Changes at the Midblastula Stage, Cell, 1982, vol. 30, no. 3, pp. 675–686.
Puck, T.T. and Steffen, J., Life Cycle Analysis of Mammalian Cells. 1. A Method for Localizing Metabolic Events within Cell Cycle and Its Application to the Action of Colcemide and Sublethal Doese of X-Irradiation, Biophys. J., 1963, vol. 3, pp. 379–389.
Rieder, C.L. and Palazzo, R.E., Colcemid and Mitotic Cycle, J. Cell Sci., 1992, vol. 102, pp. 387–392.
Rott, N.N., Kletochnye tsikly v rannem embriogeneze zhivotnykh (Cell Cycles in Early Animal Development), Moscow: Nauka, 1987.
Satoh, N., “Metachronous” Cleavage and Initiation of Gastrulation in Amphibian Embryos, Devel. Growth Differ., 1977, vol. 19, no. 2, pp. 111–117.
Solnica-Krezel, L. and Driever, W., Microtubule Arrays Function during Epiboly, Development, 1994, vol. 120, pp. 2443–2455.
Strahle, U. and Jesuthasan, S., Ultraviolet Irradiation Impairs Epiboly in Zebrafish Embryos: Evidence for a Microtubule-Dependent Mechanism of Epiboly, Devel. Biol., 1993, vol. 119, pp. 909–919.
Taylor, E.W., The Mechanism of Colchicine Inhibition of Mitosis. 1. Kinetics of Inhibition and the Binding of H3-Colchicine, J. Cell Biol., 1965, vol. 25, no. 1, pp. 145–160.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.I. Efremov, G.M. Gluzdikova, E.V. Mukhachev, 2007, published in Ontogenez, 2007, Vol. 38, No. 5, pp. 372–379.
Rights and permissions
About this article
Cite this article
Efremov, V.I., Gluzdikova, G.M. & Mukhachev, E.V. Development of the model of cell cycle synchronization in early embryos of Danio rerio (Teleostei). Russ J Dev Biol 38, 310–316 (2007). https://doi.org/10.1134/S1062360407050050
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1134/S1062360407050050