Abstract
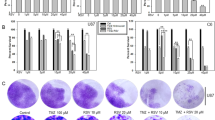
The efficacy of Temozolomide (TMZ)-based chemotherapy in human malignancy is limited by the occurrence of innate and acquired drug resistance. Hence, in the present study, we tend to evaluate the effects of Galangin (GLN) combined with TMZ on proliferation and migration and its underlying mechanisms in the U251 cells of glioblastoma. MTT assay and cell death ELISA kit evaluated cellular proliferation and apoptosis, respectively. The mRNA levels of invasive genes were evaluated using the qRT-PCR. We also applied DCFH-DA fluorescence dye to detect reactive oxygen species (ROS) formation and activities of antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, and glutathione S-transferase) in U251 cells. GLN considerably increased the cytotoxic effects of TMZ on the proliferation of U251 cells (P < 0.01). Also, this co-treatment potentiated the apoptosis rate in these tumor cells (P < 0.001). This outcome is achieved by decreasing metastatic and invasive genes (VEGF-A, MMP2, MMP9, and EpCAM). Moreover, ROS levels were increased (P < 0.01), and antioxidant enzymes’ expression levels were significantly decreased in the co-treatment group compared to the alone treatment with GLN or TMZ in U251 cells. Findings demonstrate a novel mechanism by which GLN enhances the cytotoxic effects of TMZ on tumor cells. This combinational therapy might promise a therapeutic regimen for improving the clinical efficacy in glioma patients.







Similar content being viewed by others
REFERENCES
Arafa, M.G., Ghalwash, D., El-Kersh, D.M., and Elmazar, M.M., Propolis-based niosomes as oromuco-adhesive films: a randomized clinical trial of a therapeutic drug delivery platform for the treatment of oral recurrent aphthous ulcers, Sci. Rep., 2018, vol. 8, p. 18056. https://doi.org/10.1038/s41598-018-37157-7
Bazavar, M., Fazli, J., Valizadeh, A., Ma, B., Mohammadi, E., Asemi, Z., Alemi, F., Maleki, M., Xing, S., and Yousefi, B., miR-192 enhances sensitivity of methotrexate drug to MG-63 osteosarcoma cancer cells, Pathol. Res. Pract., 2020, vol. 216, p. 153176. https://doi.org/10.1016/j.prp.2020.153176
Burri, S.H., Gondi, V., Brown, P.D., and Mehta, M.P., The evolving role of tumor treating fields in managing glioblastoma: guide for oncologists, Am. J. Clin. Oncol., 2018, vol. 41, pp. 191–196. https://doi.org/10.1097/coc.0000000000000395
Çıtışlı, V., Dodurga, Y., Eroğlu, C., Seçme, M., Avcı, Ç, B., and Şatıroğlu-Tufan, N. L., Temozolomide may induce cell cycle arrest by interacting with URG4/URGCP in SH-SY5Y neuroblastoma cells, Tumour Biol., 2015, vol. 36, pp. 6765–6772. https://doi.org/10.1007/s13277-015-3373-7
Filippi-Chiela, E.C., Thomé, M.P., Bueno e Silva, M.M., Pelegrini, A.L., Ledur, P.F., Garicochea, B., Zamin, L.L., and Lenz, G., Resveratrol abrogates the temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the temozolomide-induced senescence in glioma cells, BMC Cancer, 2013, vol. 13, p. 147. https://doi.org/10.1186/1471-2407-13-147
Franceschi, E., Omuro, A.M., Lassman, A.B., Demopoulos, A., Nolan, C., and Abrey, L.E., Salvage temozolomide for prior temozolomide responders, Cancer, 2005, vol. 104, pp. 2473–2476. https://doi.org/10.1002/cncr.21564
Han, M.A., Lee, D.H., Woo, S.M., Seo, B.R., Min, K.J., Kim, S., Park, J.W., Kim, S.H., Choi, Y.H., and Kwon, T.K., Galangin sensitizes TRAIL-induced apoptosis through down-regulation of anti-apoptotic proteins in renal carcinoma Caki cells, Sci. Rep., 2016, vol. 6, p. 18642. https://doi.org/10.1038/srep18642
Knizhnik, A.V., Roos, W.P., Nikolova, T., Quiros, S., Tomaszowski, K.H., Christmann, M., and Kaina, B., Survival and death strategies in glioma cells: autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage, PLoS One, 2013, vol. 8, article ID e55665. https://doi.org/10.1371/journal.pone.0055665
Kong, Y., Feng, Z., Chen, A., Qi, Q., Han, M., Wang, S., Zhang, Y., Zhang, X., Yang, N., Wang, J., Huang, B., Zhang, Q., Xiang, G., Li, W., Zhang, D., Wang, J., and Li, X., The natural flavonoid galangin elicits apoptosis, pyroptosis, and autophagy in glioblastoma, Front. Oncol., 2019, vol. 9, p. 942. https://doi.org/10.3389/fonc.2019.00942
Lee, H.S., Park, B.S., Kang, H.M., Kim, J.H., Shin, S.H., and Kim, I.R., Role of luteolin-induced apoptosis and autophagy in human glioblastoma cell lines, Medicina (Kaunas), 2021, vol. 57. https://doi.org/10.3390/medicina57090879
Li, X., Wang, Y., Xiong, Y., Wu, J., Ding, H., Chen, X., Lan, L., and Zhang, H., Galangin induces autophagy via deacetylation of LC3 by SIRT1 in HepG2 cells, Sci. Rep., 2016, vol. 6, p. 30496. https://doi.org/10.1038/srep30496
Lin, C.J., Lee, C.C., Shih, Y.L., Lin, C.H., Wang, S.H., Chen, T.H., and Shih, C.M., Inhibition of mitochondria- and endoplasmic reticulum stress-mediated autophagy augments temozolomide-induced apoptosis in glioma cells, PLoS One, 2012a, vol. 7, article ID e38706. https://doi.org/10.1371/journal.pone.0038706
Lin, C.J., Lee, C.C., Shih, Y.L., Lin, T.Y., Wang, S.H., Lin, Y.F., and Shih, C.M., Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy, Free Radical Biol. Med., 2012b, vol. 52, pp. 377–391. https://doi.org/10.1016/j.freeradbiomed.2011.10.487
Marta, G.N., Moraes, F.Y., Feher, O., Vellutini, E.d.A.S., Pahl, F.H., Gomes, M.d.Q.T., Cardoso, A.C.C., Neville, I.S., Hanna, S.A., Palhares, D.M.F., Teixeira, M.J., Maldaun, M.V.C., and Pereira, A.A.L., Social determinants of health and survival on Brazilian patients with glioblastoma: a retrospective analysis of a large populational database, Lancet Reg. Health Am., 2021, vol. 4. https://doi.org/10.1016/j.lana.2021.100066
Miller, K.D., Ostrom, Q.T., Kruchko, C., Patil, N., Tihan, T., Cioffi, G., Fuchs, H.E., Waite, K.A., Jemal, A., Siegel, R.L., and Barnholtz-Sloan, J.S., Brain and other central nervous system tumor statistics, 2021, CA Cancer J. Clin., 2021, vol. 71, pp. 381–406. https://doi.org/10.3322/caac.21693
Mirabdaly, S., Elieh Ali Komi, D., Shakiba, Y., Moini, A., and Kiani, A., Effects of temozolomide on U87MG glioblastoma cell expression of CXCR4, MMP2, MMP9, VEGF, anti-proliferatory cytotoxic and apoptotic properties, Mol. Biol. Rep., 2020, vol. 47, pp. 1187–1197. https://doi.org/10.1007/s11033-019-05219-2
Nam, J.Y. and de Groot, J.F., Treatment of glioblastoma, J. Oncol. Pract., 2017, vol. 13, pp. 629–638. https://doi.org/10.1200/jop.2017.025536
Perry, J.R., Laperriere, N., O’Callaghan, C.J., Brandes, A.A., Menten, J., Phillips, C., Fay, M., Nishikawa, R., Cairncross, J.G., Roa, W., Osoba, D., Rossiter, J.P., Sahgal, A., Hirte, H., Laigle-Donadey, F., Franceschi, E., Chinot, O., Golfinopoulos, V., Fariselli, L., Wick, A., Feuvret, L., Back, M., Tills, M., Winch, C., Baumert, B.G., Wick, W., Ding, K., and Mason, W.P., Short-course radiation plus temozolomide in elderly patients with glioblastoma, N. Engl. J. Med., 2017, vol. 376, pp. 1027–1037. https://doi.org/10.1056/NEJMoa1611977
Piret, B., Schoonbroodt, S., and Piette, J., The ATM protein is required for sustained activation of NF-kappaB following DNA damage, Oncogene, 1999, vol. 18, pp. 2261–2271. https://doi.org/10.1038/sj.onc.1202541
Shi, Y., Jiang, J., Cui, Y., Chen, Y., Dong, T., An, H., and Liu, P., MSH6 aggravates the hypoxic microenvironment via regulating HIF1A to promote the metastasis of glioblastoma multiforme, DNA Cell Biol., 2021, vol. 40, pp. 93–100. https://doi.org/10.1089/dna.2020.5442
Sinha, R., Srivastava, S., Joshi, A., Joshi, U.J., and Govil, G., In-vitro anti-proliferative and antioxidant activity of galangin, fisetin and quercetin: role of localization and intermolecular interaction in model membrane, Eur. J. Med. Chem., 2014, vol. 79, pp. 102–109. https://doi.org/10.1016/j.ejmech.2014.04.002
Tan, A.C., Ashley, D.M., López, G.Y., Malinzak, M., Friedman, H.S., and Khasraw, M., Management of glioblastoma: state of the art and future directions, CA Cancer J. Clin., 2020, vol. 70, pp. 299–312. https://doi.org/10.3322/caac.21613
Terzis, A.J., Thorsen, F., Heese, O., Visted, T., Bjerkvig, R., Dahl, O., Arnold, H., and Gundersen, G., Proliferation, migration and invasion of human glioma cells exposed to paclitaxel (Taxol) in vitro, Br. J. Cancer, 1997, vol. 75, pp. 1744–1752. https://doi.org/10.1038/bjc.1997.298
Tomicic, M.T., Meise, R., Aasland, D., Berte, N., Kitzinger, R., Krämer, O.H., Kaina, B., and Christmann, M., Apoptosis induced by temozolomide and nimustine in glioblastoma cells is supported by JNK/c-Jun-mediated induction of the BH3-only protein BIM, Oncotarget, 2015, vol. 6, pp. 33755–33768. https://doi.org/10.18632/oncotarget.5274
Wang, Y., Wu, J., Lin, B., Li, X., Zhang, H., Ding, H., Chen, X., Lan, L., and Luo, H., Galangin suppresses HepG2 cell proliferation by activating the TGF-β receptor/Smad pathway, Toxicology, 2014, vol. 326, pp. 9–17. https://doi.org/10.1016/j.tox.2014.09.010
Yang, C.C., Lin, C.C., Hsiao, L.D., and Yang, C.M., Galangin inhibits thrombin-induced MMP-9 expression in SK-N-SH cells via protein kinase-dependent NF-κB phosphorylation, Int. J. Mol. Sci., 2018, vol. 19. https://doi.org/10.3390/ijms19124084
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Yiyun Li, Wan, Y., Yu, N. et al. Galangin (GLN) Promotes Temozolomide-Induced Apoptosis in Glioma Cells. Biol Bull Russ Acad Sci 49, 580–587 (2022). https://doi.org/10.1134/S1062359022060085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022060085