Abstract—
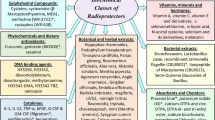
A classification of radiomitigators, i.e., antiradiation agents for prevention or reduction of the severity of clinical manifestations of acute radiation syndrome and its delayed and long-term clinical consequences, has been proposed. In the classification proposed, radiomitigators used for prophylaxis and treatment of the immediate and remote clinical manifestations of acute radiation syndrome are divided into the following main groups: means (drugs) for treatment of the bone marrow syndrome; means for treatment of the gastrointestinal syndrome; means for treatment of local and subtotal manifestations of radiation injuries; means for prevention and treatment of delayed and long-term effects of irradiation. A list and brief characteristics of the most promising radiomitigators from the groups of cytokines, growth factors, antioxidants, immunomodulators, steroid hormone analogues, and apoptosis blockers are given.

Similar content being viewed by others
REFERENCES
Hall, E.J. and Giaccia, A.J., Radiobiology for the Radiologist, Philadelphia: Lippincott Williams and Wilkins, 2006.
Vasin, M.V., Classification of radioprotective agents as a reflection of the current state and prospects of development of radiation pharmacology, Radiats. Biol. Radioekol., 2013, vol. 53, no. 5, pp. 459–467.
Grebenyuk, A.N., Legeza, V.I., Nazarov, V.B., and Timoshevskii, A.A., Meditsinskie sredstva profilaktiki i terapii radiatsionnykh porazhenii (Health Care Aids of Prevention and Treatment of Radiation Injuries), St. Petersburg: Foliant, 2011.
Grebenyuk, A.N., Legeza, V.I., and Tarumov, R.A., Radiomitigators: prospects for use in the medical radiation protection system, Voen.-Med. Zh., 2014, vol. 335, no. 6, pp. 39–43.
Vasin, M.V., Protivoluchevye svoistva lekarstvennykh sredstv (Antiradiation Properties of Drugs), Moscow: RMAPO, 2010.
Trog, D., Bank, P., Wendt, T.G., and Beleites, E., Daily amifostine given concomitantly to chemoradiation in head and neck cancer, Strahlentherapie Onkologie, 1999, vol. 175, no. 9, pp. 444–449.
Il’in, L.A., Rudnyi, N.M., Suvorov, N.N., et al., Indralin—radioprotektor ekstrennogo deistviya. Protivoluchevye svoistva, farmakologiya, mekhanizm deistviya, klinika (Indralin—Radioprotectant of Emergency Action: Radioprotective Properties, Pharmacology, Mechanism of Action, and Clinical Use), Moscow: Min. Zdrav. Ross. Fed., 1994.
Vasin, M.V., Ushakov, I.B., Kovtun, V.Yu., et al., The characteristic of the radioprotective properties of a radioprotectant B-190 at its administration after radiation, Radiats. Biol. Radioekol., 2008, vol. 48, no. 6, pp. 730–733.
Legeza, V.I. and Chigareva, N.G., Means and methods of early pathogenetic therapy of radiation injuries, Med. Katastrof, 1999, vol. 26, no. 2, pp. 41–45.
Andrushchenko, V.N., Ivanov, A.A., and Mal’tsev, V.N., Radiation-protective action of microbial substances, Radiats. Biol. Radioekol., 1996, vol. 36, no. 2, pp. 195–207.
Ivanov, A.A., Ivanova, A.S., Ulanova, N.M., et al., Antiradiation effects of vaccine Grippol, Radiats. Biol. Radioekol., 2010, vol. 50, no. 1, pp. 52–57.
Chertkov, K.S., Treatment and prevention of acute radiation sickness in the conditions of mass destruction (according to experimental data), in Radiatsionnaya meditsina (Radiation Medicine), Il’in, L.A., Ed., Moscow: IzdAT, 2001, vol. 2.
Chertkov, K.S., Davydova, S.A., and Nesterova, T.A., Efficiency of polysaccharide translam for early treatment of acute radiation illness, Radiats. Biol. Radioekol., 1999, vol. 39, no. 5, pp. 572–577.
Burdelya, L.G., Krivokrysenko, V.I., Tallant, T.C., et al., An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models, Science, 2008, vol. 320, no. 5873, pp. 226–230.
Grebenyuk, A.N., Aksenova, N.V., Petrov, A.V., et al., Obtaining different variants of recombinant flagellin and assessment of their radioprotective efficiency, Vestn. Ross. Voen.-Med. Akad., 2013, no. 3 (43), рр. 75–80.
Ketlinskii, S.A. and Simbirtsev, A.S., Tsitokiny (Cytokines), St. Petersburg: Foliant, 2008.
Simbirtsev, A.S., Tsitokiny v patogeneze i lechenii zabolevanii cheloveka (Cytokines in the Pathogenesis and Treatment of Human Diseases), St. Petersburg: Foliant, 2018.
Grebenyuk, A.N. and Legeza, V.I., Protivoluchevye svoistva interleikina-1 (Antiradiation Properties of Interleukin-1), St. Petersburg: Foliant, 2012.
Rozhdestvenskii, L.M., Korovkina, E.P., and Deshevoi, Yu.B., Recombinant human interleukine-1beta (betaleukine) usage for acute radiation sickness of severe degree treatment at canines, Radiats. Biol. Radioekol., vol. 48, no. 2, pp. 185–194.
Wang, C., Zhang, B., Wang, S., et al., Recombinant human thrombopoietin promotes hematopoietic reconstruction after severe whole body irradiation, Sci. Rep., 2015, no. 5, pp. 12–24.
Kuter, D.J., New thrombopoietic growth factors, Blood, 2007, vol. 109, no. 11, pp. 4607–4616.
Satyamitra, M., Lombardini, E., Graves, J., et al., A TPO receptor agonist, ALXN4100TPO, mitigates radiation-induced lethality and stimulates hematopoiesis in CD2F1 mice, Radiat. Res., 2011, vol. 175, no. 6, pp. 746–758.
Neelis, K.J., Dubbelman, Y.D., Qingliang, L., et al., Simultaneous administration of TPO and G-CSF after cytoreductive treatment of Rhesus monkeys prevents thrombocytopenia, accelerates platelet and reconstitution, alleviates neutropenia, and promotes the recovery of immature bone marrow cells, Exp. Hematol., 1997, vol. 25, no. 10, pp. 1084–1093.
Neelis, K.J., Hartong, S.C., Egeland, T., et al., The efficacy of single dose administration of thrombopoietin with co-administration of either granulocyte/macrophage or granulocyte colony stimulated factor in myelosuppressed Rhesus monkeys, Blood, 1997, vol. 90, no. 7, pp. 2565–2573.
Herodin, F., Bourin, P., Mayol, J.-F., et al., Short-term injection of antiapoptotic cytokine combinations soon after lethal γ-irradiation promotes survival, Blood, 2003, vol. 101, no. 7, pp. 2609–2616.
Whitnall, M.H., Wilhelmsen, C.L., McKinney, L.-A., et al., Radioprotective efficacy and acute toxicity of 5-androstenediol after subcutaneous or oral administration in mice, Immunopharmacol. Immunotoxicol., 2002, vol. 24, no. 4, pp. 595–626.
Weiss, J.F. and Landauer, M.R., Radioprotection by antioxidants, Ann. N.Y. Acad. Sci., 2000, vol. 899, pp. 44–60.
Sventitskaya, A.M., Nikiforov, A.S., Ivanov, I.M., et al., Comparative evaluation of therapeutic efficacy of somatotropin, growth hormone-releasing factors, and hematopoietic cytokines in experiments on irradiated mice, Radiats. Biol. Radioekol., 2017, vol. 57, no. 6, pp. 621–629.
Tarumov, R.A., Basharin, V.A., and Grebenyuk, A.N., Radioprotective properties of modern antioxidants, Medline. Ru, 2012, vol. 13, pp. 682–700.
Landauer, M.R., Srinivasan, V., and Seed, T.M., Genistein treatment protects mice from ionizing radiation injury, J. Appl. Toxicol., 2003, vol. 23, no. 6, pp. 379–385.
Bhatia, A.L., Gaur, A., and Sharma, A., Radiation protection by an isoflavone, genistein: a study on the survivability of mice, Nucl. Techn. Radiat. Prot., 2007, vol. 22, no. 1, pp. 34–39.
Davis, T.A., Clarke, T.K., and Landauer, M.R., Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival, Int. J. Radiat. Biol., 2007, vol. 83, no. 3, pp. 141–151.
Grebenyuk, A.N., Tarumov, R.A., Basharin, V.A., et al., Experimental evaluation of the effect of synthetic genistein on hematologic indices and cytokine status of irradiated rats, Radiats. Biol. Radioekol., 2015, vol. 55, no. 2, pp. 160–168.
Grebenyuk, A.N., Tarumov, R.A., Basharin, V.A., and Kovtun, V.Yu., Experimental evaluation of radioprotective efficacy of synthetic genistein on criteria of glutathione system and lipid peroxidation in erythrocytes of peripheral blood in irradiated rats, Radiats. Biol. Radioekol., 2015, vol. 55, no. 5, pp. 501–506.
Davis, T.A., Mungunsukh, O., Zins, S., et al., Genistein induces radioprotection by hematopoietic stem cell quiescence, Int. J. Radiat. Biol., 2008, vol. 84, no. 9, pp. 713–726.
Vijayalaxmi, Reiter, R.J., Tan, D.X., et al., Melatonin as a radioprotective agent: a review, Int. J. Radiat. Oncol. Biol. Phys., 2004, vol. 59, no. 3, pp. 639–653.
Pikalova, L.V., Legeza, V.I., and Gorbunov, V.A., The experimental evaluation of influence of exogenous melatonin on the genetic damage induced by radiation exposure, Radiats. Biol. Radioekol., 2013, vol. 53, no. 5, pp. 500–505.
Mil’, E.M., Albantova, A.A., and Burlakova, E.B., Impact of the antioxidant Phenozan and low dose radiation on the level of p53 and BCL-2 proteins of mice different lines, Radiats. Biol. Radioekol., 2010, vol. 50, no. 1, pp. 58–64.
Jagetia, G.C., Radioprotective potential of plants and herbs against the effects of ionizing radiation, J. Clin. Biochem. Nutr., 2007, vol. 40, no. 2, pp. 74–81.
Jagetia, G.C. and Baliga, M.S., The evolution of the radioprotective effect of chyavanaprasha (an ayurvedic rasayana drug) in mice exposed to lethal dose of gamma-radiation: a preliminary study, Phytother. Res., 2004, vol. 18, no. 1, pp. 14–18.
Krishna, A. and Kumar, A., Evaluation of radioprotective effects of rajgira (Amaranthus paniculatus) extract in Swiss albino mice, J. Radiat. Res., 2005, vol. 46, no. 2, pp. 233–239.
Park, E., Ahn, G., Yun, J.S., et al., Dieckol rescues mice from lethal irradiation by accelerating hemopoiesis and curtailing immunosuppression, Int. J. Radiat. Biol., 2010, vol. 86, no. 10, pp. 848–859.
Maurya, D.K., Salvi, V.P., and Nair, C.K., Radiation protection of DNA by ferulic acid under in vitro and in vivo conditions, Mol. Cell Biochem., 2005, vol. 280, nos. 1–2, pp. 209–217.
Mishra, K., Srivastava, P.S., and Chaudhury, N.K., Sesamol as a potential radioprotective agent: in vitro studies, Radiat. Res., 2011, vol. 176, no. 5, pp. 613–623.
Singh, P.K., Wise, S.Y., Ducey, E.J., et al., α-Tocopherol succinate protects mice against radiation-induced gastrointestinal injury, Radiat. Res., 2012, vol. 177, no. 2, pp. 133–145.
Singh, V.K., Beattie, L.A., and Seed, T.M., Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures, J. Radiat. Res., 2013, vol. 54, no. 6, pp. 973–988.
Gudkov, S.V., Gudkova, O.Y., Chernikov, A.V., and Bruscov, V.I., Protection of mice against X-ray injuries by the post-irradiation administration of guanosine and inosine, Int. J. Radiat. Biol., 2009, vol. 85, no. 2, pp. 116–125.
Soligenix, Inc., OrbeShield® for Gastrointestinal Acute Radiation Syndrome (GI ARS), 2018. www.soligenix.com/pipeline/vaccinesbiodefense/orbeshield-for-gastrointestinal-acute-radiation-syndrome-gi-ars/. Accessed June 28, 2018.
Gluzman-Poltorak, Z., Mendonca, S.R., Vainstein, V., et al., Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation, J. Hematol. Oncol., 2014, vol. 31, p. 7. https://doi.org/10.1186/1756-8722-7-31
Finch, P.W., Mark, CrossL.J., McAuley, D.F., and Farrell, C.L., Palifermin for the protection and regeneration of epithelial tissues following injury: new findings in basic research and pre-clinical models, J. Cell. Mol. Med., 2013, vol. 17, no. 9, pp. 1065–1087.
Burnett, A.F., Biju, P.G., Lui, H., and Hauer-Jensen, M., Oral interleukin 11 as a countermeasure to lethal total-body irradiation in a murine model, Radiat. Res., 2013, vol. 180, no. 6, pp. 595–602.
Koukourakis, M.I., Radiation damage and radioprotectants: new concepts in the era of molecular medicine, Br. J. Radiol., 2012, vol. 85, no. 1012, pp. 313–330.
Antushevich, A.A., Antonov, V.G., Grebenyuk, A.N., et al., Pathophysiological basis of glutoxim efficiency as an accompanying drug in radiation therapy of oropharyngeal cancer, Vestn. Ross. Voen.-Med. Akad., 2013, no. 3 (43), pp. 32–37.
Yartseva, A.A., Stepanov, A.V., Grebenyuk, A.N., and Antushevich, A.E., Effect of molixan on the oral microbiocenosis after combined chemoradiation exposure, Med.-Biol. Sots.-Psikhol. Probl. Bezop. Chrezv. Situats., 2014, no. 1, pp. 57–63.
Yartseva, A.A., Moroz, B.T., Grebenyuk, A.N., et al., The effectiveness of molixan as a means of correction of chemoradiation therapy negative manifestations in patients with oropharyngeal cancer, Radiats. Biol. Radioekol., 2014, vol. 54, no. 3, pp. 265–272.
Yartseva, A.A., Antushevich, A.E., and Grebenyuk, A.N., Experimental study of the mechanisms of hemostimulating activity of organic salts of glutathione disulfide and inosine under acute radiation exposure, Med.-Biol. Sots.-Psikhol. Probl. Bezop. Chrezv. Situats., 2016, no. 1, pp. 79–84.
Prasanna, P.G.S., Narayanan, D., Hallett, K., et al., Radioprotectors and radiomitigators for improving radiation therapy: the small business innovation research (SBIR) gateway for accelerating clinical translation, Radiat. Res., 2015, vol. 184, no. 3, pp. 235–248.
Zharikov, A.A., Terekhov, O.V., and Pasov, V.V., Treatment of patients with late radiation damage of pelvic organs with the use of the drug rexod, Onkologiya, 2013, no. 5, pp. 26–30.
Carsten, R.E., Bachand, A.M., Bailey, S.M., and Ullrich, R.L., Resveratrol reduces radiation-induced chromosome aberration frequencies in mouse bone marrow cells, Radiat. Res., 2008, vol. 169, no. 6, pp. 633–638.
Kulkarni, S.S. and Cantó, C., The molecular targets of resveratrol, Biochim. Biophys. Acta, 2015, vol. 1852, no. 6, pp. 1114–1123.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Novikova
Rights and permissions
About this article
Cite this article
Legeza, V.I., Grebenyuk, A.N. & Drachev, I.S. Radiomitigators: Classification, Pharmacological Properties, and Application Prospects. Biol Bull Russ Acad Sci 46, 1625–1632 (2019). https://doi.org/10.1134/S1062359019120045
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359019120045