Abstract—
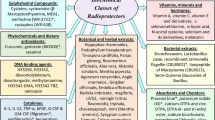
An analysis of the current status and future directions of developing medicines for the prevention and early treatment of radiation damage has been conducted. Among radioprotectors, only B-190 (Indralin) is approved for use in the Russian Federation; the main radioprotector in foreign countries is amifostine (Ethyol). Some prospects in the development of new radioprotectors are associated with inhibitors of NO-synthases from the class of N-acyl-S-alkyl-substituted isothiourea derivatives, among which chemical compounds with a pronounced radioprotective effect are found. A recombinant human interleukin-1β (Betaleukin) has been registered in Russia as a radiomitigator intended for use in the early period after accidental irradiation. The US Food and Drug Administration (FDA) has approved the investigational new drug status for seven radiomitigators, including 5-androstenediol (Neumune), genistein (BIO 300), CBLB502 (Entolimod), a kinase inhibitor ON01210 (Ex-RAD), recombinant human interleukin-12 (HemaMax), beclomethasone (OrbeShield), and a granulocyte colony-stimulating factor (G-CSF, Neupogen). Ondansetron hydrochloride dihydrate (Latran) was registered as an antiemetic drug for prevention and therapy of the primary response to radiation in Russia, and granisetron (Kytril) was registered as an antiemetic drug upon irradiation abroad. Potassium iodide, potassium ferric hexacyanoferrate (Ferrocin), diethylenetriaminepentaacetate calcium trisodium (Pentacin, calcium trisodium pentetate), and 2,3-dimercaptopropansulfonate sodium (Unithiol) are used in Russia for the prevention and treatment of damage from irradiation via incorporated radionuclides. Potassium iodide (ThyroShield), diethylenetriaminepentaacetate zinc trisodium (Zn-DTPA, Pentetate zinc trisodium), diethylenetriaminepentaacetate calcium trisodium (Ca-DTPA, Pentetate calcium trisodium), and ferric hexacyanoferrate (Prussian blue, Radiogardase) are used as decorporation agents abroad. Cytokines, vitamins, low-molecular compounds, inhibitors of apoptosis, etc., are considered promising means for the prevention and early treatment of radiation damage.
Similar content being viewed by others
REFERENCES
Grebenyuk, A.N., Legeza, V.I., Nazarov, V.B., and Timoshevskii, A.A., Meditsinskie sredstva profilaktiki i terapii radiatsionnykh porazhenii (Medicines for Prevention and Treatment of Radiation Injuries), St. Petersburg: Foliant, 2011.
Grebenyuk, A.N., Legeza, V.I., Gladkikh, V.D., et al., Prakticheskoe rukovodstvo po ispol’zovaniyu meditsinskikh sredstv protivoradiatsionnoi zashchity pri chrezvychainykh situatsiyakh i obespecheniyu imi avariinykh mediko-sanitarnykh formirovanii i regional’nykh avariinykh tsentrov (A Practical Guide to Using Medicines for Radiation Protection in Emergency Situations and Their Supply to Emergency Health Units and Regional Emergency Centers), Moscow: Kommentarii, 2015.
Il’in, L.A., Ushakov, I.B., and Vasin, M.V., Radioprotective agents in the system of radiation protection of personnel and population in case of radiation accidents, Med. Radiol. Radiats. Bezop., 2012, vol. 57, no. 3, pp. 26–31.
Legeza, V.I., Grebenyuk, A.N., and Zatsepin, V.V., Medical protection during radiation accidents: some results and lessons of the Chernobyl accident, Radiats. Biol. Radioecol., 2011, vol. 51, no. 1, pp. 70–75.
Vasin, M.V., Classification of radioprotective agents as a reflection of the current state and prospects of development of radiation pharmacology, Radiats. Biol. Radioecol., 2013, vol. 53, no. 5, pp. 459–467.
Grebenyuk, A.N., Legeza, V.I., and Tarumov, R.A., Radiomitigators: prospects for use in the system of medical radiation protection, Voen.-Med. Zh., 2014, vol. 335, no. 6, pp. 39–43.
Grebenyuk, A.N. and Legeza, V.I., Prospects of using radioprotectants to improve the efficiency of medical radiation protection of the Armed Forces, Voen.-Med. Zh., 2013, vol. 334, no. 7, рр. 46–50.
Singh, V., Newman, V.L., Romaine, P.L., et al., Radiation countermeasure agents: an update (2011–2014), Expert.Opin. Ther., 2014, vol. 24, no. 11, pp. 1229–1255.
Il’in, L.A., Rudnyi, N.M., Suvorov, N.N., et al., Indralin—radioprotektor ekstrennogo deistviya. Protivoluchevye svoistva. Farmakologiya, mekhanizm deistviya, klinika (Indralin—A Radioprotectant of Emergency Action: Radioprotective Properties, Pharmacology, Mechanism of Action, and Clinical Use), Moscow, 1994.
Vasin, M.V., Ushakov, I.B., Kovtun, V.Yu., et al., The characteristic of radioprotective properties of a radioprotectant B-190 at its administration after radiation, Radiats. Biol. Radioecol., 2008, vol. 48, no. 6, pp. 730–733.
Vasin, M.V., Ushakov, I.B., Kovtun, V.Yu., et al., Radioprotective properties of a radioprotector of emergency action indralin at its administration after irradiation in conditions of local shielding of a rat abdomen, Radiats. Biol. Radioecol., 2008, vol. 48, no. 2, pp. 199–202.
Vasin, M.V., Ushakov, I.B., Kovtun, V.Yu., et al., Radioprotectant indralin at early and late manifestations of local radiation injuries, Vopr. Onkol., 2016, vol. 62, no. 3, pp. 406–412.
Shashkov, V.S., Efimov, V.I., Vasin, M.V., et al., Indralin as a new effective radioprotectant under exposure to high-energy protons, Aviakosm. Ekol. Med., 2010, vol. 44, no. 1, pp. 15–20.
Vasin, M.V., Antipov, V.V., Komarova, S.N., et al., Radioprotective properties of indralin combined with cystamine and mexamine, Radiats. Biol. Radioecol., 2011, vol. 51, no. 2, pp. 243–246.
Grebenyuk, A.N., Zatsepin, V.V., Aksenova, N.V., et al., The influence of consecutive application of B-190 preparation and interleukin-1beta on survival rate and bone marrow hematopoiesis of irradiated mice, Radiats. Biol. Radioecol., 2010, vol. 50, no. 4, pp. 475–480.
Grebenyuk, A.N., Aksenova, N.V., Zatsepin, V.V., et al., The influence of consecutive application of radioprotector B-190 and interleukin-1beta on changes of number of peripheral blood leucocytes and functional status of neutrophils of irradiated mice, Radiats. Biol. Radioecol., 2013, vol. 53, no. 3, pp. 290–295.
Vasin, M.V., Ushakov, I.B., Kovtun, V.Yu., et al., The influence of combined application of quercetin and indralin on post-irradiation repair of hematopoiesis in acute radiation injury, Radiats. Biol. Radioecol., 2011, vol. 51, no. 2, pp. 247–251.
Miroshnichenko, Yu.V., Boyarintsev, V.V., Grebenyuk, A.N., et al., The use of modern first-aid kits and bags during liquidation of aftermaths of emergencies, Kreml. Med., Klin. Vestn., 2013, no. 2, pp. 176–181.
Weiss, J.F. and Landauer, M.R., History and development of radiation-protective agents, Int. J. Radiat. Biol., 2009, vol. 85, no. 7, pp. 539–573.
Wasserman, T.H. and Brizel, D.M., The role of amifostine as a radioprotector, Oncology (Williston Park, N.Y.), 2001, vol. 15, no. 10, pp. 1349–1354.
Bourhis, J. and Rosine, D., Radioprotective effect of amifostine in patients with head and neck squamous cell carcinoma, Semin. Oncol., 2002, vol. 29, no. 6, Suppl. 19, pp. 61–62.
Kouvaris, J.R., Kouloulias, V.E., and Vlahos, L.J., Amifostine: the first selective-target and broad-spectrum radioprotector, Oncologist, 2007, vol. 12, no. 6, pp. 738–747.
Karacetin, D., Yücel, B., Leblebicioğlu, B., et al., A randomized trial of amifostine as radioprotector in the radiotherapy of head and neck cancer, J. BUON, 2004, vol. 9, no. 1, pp. 23–26.
Kuna, P., Dostal, M., Neruda, O., et al., Acute toxicity and radioprotective effects of amifostine (WR-2721) or cystamine in single whole body fission neutrons irradiated rats, J. Appl. Biomed., 2004, vol. 2, no. 1, pp. 43–49.
Rosen, E.M., Day, R., and Singh, V.K., New approaches to radiation protection, Front. Oncol., 2014, vol. 4, p. 381. https://doi.org/10.3389/fonc.2014.00381
Koukourakis, M.I., Radiation damage and radioprotectants: new concepts in the era of molecular medicine, Br. J. Radiol., 2012, vol. 85, no. 1012, pp. 313–330.
Gladkikh, V.D., Balandin, N.V., Belovolov, A.Yu., et al., Sostoyanie i perspektivy razvitiya sredstv profilaktiki i lecheniya radiatsionnykh porazhenii (The State and Prospects of Development of the Means of Prevention and Treatment of Radiation Injuries), Gladkikh, V.D., Ed., Moscow: Kommentarii, 2016.
Filimonova, M.V., Proskuryakov, S.Ya., Shevchenko, L.I., et al., Radioprotective properties of isothiourea derivatives with NO-inhibitory mechanism of action, Radiats. Biol. Radioecol., 2012, vol. 52, no. 6, pp. 593–601.
Filimonova, M.V., Shevchenko, L.I., Trofimova, T.P., et al., On the mechanism of radioprotective effect of NO-synthase inhibitors, Radiats. Biol. Radioecol., 2014, vol. 54, no. 5, pp. 500–506.
Filimonova, M.V., Shevchenko, L.I., Makarchuk, V.M., et al., Radioprotective properties of NO-synthase inhibitor T1023: I. Indicators of radioprotective activity and interaction with other radioprotectors, Radiats. Biol. Radioecol., 2015, vol. 55, no. 3, pp. 250–259.
Filimonova, M.V., Ul’yanenko, S.E., Shevchenko, L.I., et al., Radioprotective properties of NO-synthase inhibitor T1023: II. The ability for selective protection of normal tissues during radiotherapy of tumors, Radiats. Biol. Radioecol., 2015, vol. 55, no. 3, pp. 260–266.
Filimonova, M.V., Samsonova, A.S., Korneeva, T.S., et al., The study of the ability of the new inhibitor of nitric oxide synthases INOS1 to selectively protect normal tissues in Ehrlich carcinoma radiation therapy model, Radiats.Risk, 2018, vol. 27, no. 2, pp. 37–45.
Makarchuk, V.M., Filimonova, M.V., Izmest’eva, O.S., et al., Radioprotective properties of NO-synthase inhibitor T1023: III. Mechanisms of radioprotective action in vivo, Radiats. Biol. Radioecol., 2016, vol. 56, no. 6, pp. 590–597.
Ketlinskii, S.A. and Simbirtsev, A.S., Tsitokiny (Cytokines), St. Petersburg: Foliant, 2008.
Simbirtsev, A.S., Interleikin-1. Fiziologiya. Patologiya. Klinika (Interleukin-1. Physiology. Pathology. Clinical Use), St. Petersburg: Foliant, 2011.
Lebedev, V.G., Moroz, B.B., Deshevoi, Yu.B., et al., Study of mechanisms of anti-irradiation effects of interleukin-1beta in long-term bone marrow cultures, Radiats. Biol. Radioecol., 2002, vol. 42, no. 1, pp. 60–64.
Aksenova, N.V., Grebenyuk, A.N., Ketlinskii, S.A., et al., Radioprotective activity of recombinant interleukin-1β with respect to hematopoietic progenitor cells, Med. Immunol., 2003, vol. 5, nos. 5–6, pp. 621–624.
Rozhdestvenskii, L.M., Interleukin-1—central proinflammatory cytokine of pleiotropic action in the aspect of treatment of radiation injuries in experiments and in clinical practice, Med. Radiol. Radiats. Bezop., 2001, vol. 46, no. 4, pp. 5–11.
Grebenyuk, A.N., Sarkisyan, K.G., and Timoshevskii, A.A., Radioprotective properties of the interleukin-1, Vestn. Ros. Voen.-Med. Akad., 2005, no. 1 (13), pp. 44–53.
Rozhdestvenskii, L.M., Deshevoi, Yu.B., Lebedev, V.G., and Nesterova, T.N., Dependence of the therapeutic effectiveness of interleukin-1beta on the time of the drug administration after irradiation of mice, Radiats. Biol. Radioecol., 2002, vol. 42, no. 1, pp. 65–69.
Rozhdestvenskii, L.M., Korovkina, E.P., and Deshevoi, Yu.B., The use of recombinant human interleukin-1beta (betaleukin) for the emergency treatment of severe acute radiation sickness in dogs, Radiats. Biol. Radioecol., 2008, vol. 48, no. 2, pp. 185–194.
Grebenyuk, A., Zatsepin, V., Aksenova, N., and Timoshevsky, A., Effects of early therapeutic administration of interleukin-1beta on survival rate and bone marrow haemopoiesis in irradiated mice, Acta Medica (Hradec Kralove), 2010, vol. 53, no. 4, pp. 221–224.
Grebenyuk, A.N., Konev, V.V., and Timoshevskii, A.A., Experimental substantiation of the use of recombinant interleukin-1β to correct the effects of fractionated irradiation, Med. Immunol., 2005, vol. 7, nos. 5–6, pp. 605–611.
Vorobyeva, N.Yu., Grekhova, A.K., Trubitsina, K.Yu., et al., Interleukin-1β can reduce manifestations of delayed effects of prolonged exposure to low-intensity γ-radiation, Bull. Exp. Biol. Med., 2015, vol. 160, no. 4, pp. 470–473.
Rozhdestvenskii, L.M., Shlyakova, T.G., Trubitsina, K.Yu., et al., Prophylactic use of antiradiation agents in mice at low-intensity γ-irradiation, Radiats. Biol. Radioecol., 2017, vol. 57, no. 6, pp. 608–620.
Legeza, V.I., Seleznev, A.B., Zargarova, N.I., and Kondakov, A.Yu., Experimental study of therapeutic and prophylactic use of interleukin-1β (betaleukin) in combined radiation injuries, Med.-Biol. Sots.-Psikhol. Probl. Bezop. Chrezvych. Situats., 2010, no. 4, pp. 41–45.
Budagov, R.S. and Ul’yanova, L.P., Prospects for using early treatment of acute radiation sickness means in the conditions of the development of combined radiation-thermal injuries, Med. Radiol. Radiats. Bezop., 2002, vol. 47, no. 6, pp. 8–14.
Legeza, V.I., Grebenyuk, A.N., and Boyarintsev, V.V., Kombinirovannye radiatsionnye porazheniya i ikh komponenty (Combined Radiation Injuries and Their Components), St. Petersburg: Foliant, 2015.
Legeza, V.I., Grebenyuk, A.N., and Zargarova, N.I., On the effectiveness of using radioprotective of different mechanism of action in patients with injuries typical for radiation accidents (experimental study), Med.-Biol. Sots.-Psikhol. Probl. Bezop. Chrezvych. Situats., 2013, no. 1, pp. 42–47.
Legeza, V.I., Grebenyuk, A.N., Kondakov, A.Yu., and Zargarova, N.I., Comparative evaluation of healing wounds at a local and combined radiation injury in an experiment, Radiats. Biol. Radioecol., 2015, vol. 55, no. 6, pp. 584–590.
Kondakov, A.Yu., Seleznev, A.B., Stepanov, A.V., et al., Experimental study of the effect of recombinant human interleukin-1β on the manifestations of oxidative stress at local and combined radiation injuries, Radiats. Biol. Radioecol., 2017, vol. 57, no. 2, pp. 145–151.
Grebenyuk, A.N. and Legeza, V.I., Protivoluchevye svoistva interleikina-1 (Antiradiation Properties of Interleukin-1), St. Petersburg: Foliant, 2012.
Lukashin, B. and Grebenyuk, A., Radioprotective effect of heparin, Kontakt, 2011, vol. 13, no. 4, pp. 478–483.
Timoshevskii, A.A., Grebenyuk, A.N., and Kalinina, N.M., Human leukocyte response to parenteral administration of IL-1β and to subsequent irradiation of peripheral blood samples in vitro, Med. Radiol. Radiats. Bezop., 2005, vol. 50, no. 2, pp. 5–17.
Gershanovich, M.L., Filatova, L.V., Ketlinskii, S.A., and Simbirtsev, A.S., Betaleukin (human recombinant interleukin-1beta)—a new and effective stimulant and protectant of leukopoiesis in combined chemotherapy of malignant tumors, Vopr. Onkol., 2000, vol. 46, no. 3, pp. 354–360.
Gershanovich, M.L., Filatova, L.V., Ketlinsky, S.A., and Simbirtsev, A.S., Recombinant human interleukin-1β: new possibilities for prophylaxis and correction of toxic myelodepression in patients with malignant tumors. II. Phase II study of protective effect of recombinant human interleukin-1β on myelodepression induced by chemotherapy in cancer patients, Eur. Cytokine Netw., 2001, vol. 12, no. 4, pp. 671–675.
Simbirtsev, A.S., Tsitokiny v patogeneze i lechenii zabolevanii cheloveka (Cytokines in the Pathogenesis and Treatment of Human Diseases), St. Petersburg: Foliant, 2018.
Rozhdestvenskii, L.M., Antiradiation means of protection and therapy: current status, problems, and prospects, Med. Radiol. Radiats. Bezop., 2012, vol. 57, no. 5, pp. 72–82.
Singh, V., Romaine, P.L., and Seed, T.M., Medical countermeasures for radiation exposure and related injuries: characterization of medicines, FDA-approval status and inclusion into the Strategic National Stockpile, Health Phys., 2015, vol. 108, no. 6, pp. 607–630.
Singh, V.K., Romaine, P.L., Newman, V.L., and Seed, T.M., Medical countermeasures for unwanted CBRN exposures: part II radiological and nuclear threats with review of recent countermeasure patents, Expert. Opin. Ther. Pat., 2016, vol. 26, no. 12, pp. 1399–1408.
Singh, V.K., Newman, V.L., and Seed, T.M., Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review, Cytokine, 2014, vol. 71, no. 1, pp. 22–37.
Singh, V.K. and Seed, T.M., A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures, Int. J. Radiat. Biol., 2017, vol. 93, no. 9, pp. 851–869.
Singh, V.K., Hanlon, B.K., Santiago, P.T., and Seed, T.M., A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part III. Countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use, Int. J. Radiat. Biol., 2017, vol. 93, no. 9, pp. 885–906.
Neelis, K.J., Dubbelman, Y.D., Qingliang, L., et al., Simultaneous administration of TPO and G-CSF after cytoreductive treatment of Rhesus monkeys prevents thrombocytopenia, accelerates platelet and reconstitution, alleviates neutropenia, and promotes the recovery of immature bone marrow cells, Exp. Hematol., 1997, vol. 25, no. 10, pp. 1084–1093.
Neelis, K.J., Hartong, S.C., Egeland, T., et al., The efficacy of single dose administration of thrombopoietin with co-administration of either granulocyte/macrophage or granulocyte colony stimulated factor in myelosuppressed Rhesus monkeys, Blood, 1997, vol. 90, no. 7, pp. 2565–2573.
Wang, C., Zhang, B., Wang, S., et al., Recombinant human thrombopoietin promotes hematopoietic reconstruction after severe whole body irradiation, Sci. Rep., 2015, no. 5, pp. 12–24.
Satyamitra, M., Lombardini, E., Graves, J., et al., A TPO receptor agonist, ALXN4100TPO, mitigates radiation-induced lethality and stimulates hematopoiesis in CD2F1 mice, Radiat. Res., 2011, vol. 175, no. 6, pp. 746–758.
Basile, L.A., Ellefson, D., Gluzman-Poltorak, Z., et al., HemaMax™, a recombinant human interleukin-12, is a potent mitigator of acute radiation injury in mice and non-human primates, PLoS One, 2012, vol. 7, no. 2. e30434. https://doi.org/10.1371/journal.pone.0030434
Gluzman-Poltorak, Z., Mendonca, S.R., and Vainstein, V., Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation, J. Hematol. Oncol., 2014, vol. 7, p. 31.
Gluzman-Poltorak, Z., Vainstein, V., and Basile, L.A., Recombinant interleukin-12, but not granulocyte-colony stimulating factor, improves survival in lethally irradiated nonhuman primates in the absence of supportive care: evidence for the development of a frontline radiation medical countermeasure, Am. J. Hematol., 2014, vol. 89, pp. 868–873.
Gluzman-Poltorak, Z., Vainstein, V., and Basile, L.A., Association of hematological nadirs and survival in a nonhuman primate model of hematopoietic syndrome of acute radiation syndrome, Radiat. Res., 2015, vol. 184, pp. 226–230.
Herodin, F., Bourin, P., Mayol, J.-F., et al., Short-term injection of antiapoptotic cytokine combinations soon after lethal γ-irradiation promotes survival, Blood, 2003, vol. 101, no. 7, pp. 2609–2616.
Whitnall, M.H., Elliott, T.B., Harding, R.A., et al., Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice, Int. J. Immunopharmacol., 2000, vol. 22, no. 1, pp. 1–14.
Grace, M.B., Singh, V.K., Rhee, J.G., et al., 5-AED enhances survival of irradiated mice in a G-CSF-dependent manner, stimulates innate immune cell function, reduces radiation-induced DNA damage and induces genes that modulate cell cycle progression and apoptosis, J. Radiat. Res., 2012, vol. 53, no. 6, pp. 840–853.
Xiao, M., Inal, C.E., Parekh, V.I., et al., 5-Androstenediol promotes survival of gamma-irradiated human hematopoietic progenitors through induction of nuclear factor-kappaB activation and granulocyte colony-stimulating factor expression, Mol. Pharmacol., 2007, vol. 72, no. 2, pp. 370–379.
Singh, V.K., Grace, M.B., Jacobsen, K.O., et al., Administration of 5-androstenediol to mice: pharmacokinetics and cytokine gene expression, Exp. Mol. Pathol., 2008, vol. 84, no. 2, pp. 178–188.
Whitnall, M.H., Villa, V., Seed, T.M., et al., Molecular specificity of 5-androstenediol as a systemic radioprotectant in mice, Immunopharmacol. Immunotoxicol., 2005, vol. 27, no. 1, pp. 15–32.
Stickney, D.R., Dowding, C., Garsd, A., et al., 5‑Androstenediol stimulates multilineage hematopoiesis in rhesus monkeys with radiation-induced myelosuppression, Int. Immunopharmacol., 2006, vol. 6, no. 11, pp. 1706–1713.
Whitnall, M.H., Wilhelmsen, C.L., McKinney, L.-A., et al., Radioprotective efficacy and acute toxicity of 5‑androstenediol after subcutaneous or oral administration in mice, Immunopharmacol. Immunotoxicol., 2002, vol. 24, no. 4, pp. 596–626.
Kim, J.S., Jang, W.S., Lee, S., et al., A study of the effect of sequential injection of 5-androstenediol on irradiation-induced myelosuppression in mice, Arch. Pharm. Res., 2015, vol. 38, no. 6, pp. 1213–1222.
Hofer, M., Hoferova, Z., and Falk, M., Pharmacological modulation of radiation damage. Does it exist a chance for other substances than hematopoietic growth factors and cytokines? Int. J. Mol. Sci., 2017, vol. 18, no. 7. pii: E1385. https://doi.org/10.3390/ijms18071385
Gladkikh, V.D. and Kozlov, S.V., On the issue of development of innovative radioprotective drugs based on metabolites of steroid hormones, Eksp. Klin. Farmakol., 2018, vol. 81, suppl., pp. 55–56.
Shukla, P.N., Gairola, M., Mohanti, B.K., and Rath, G.K., Prophylactic beclomethasone spray to the skin during postoperative radiotherapy of carcinoma breast: a prospective randomized study, Indian J. Cancer, 2006, vol. 43, no. 4, pp. 180–184.
Fuccio, L., Guido, A., Laterza, L., et al., Randomised clinical trial: preventive treatment with topical rectal beclomethasone dipropionate reduces post-radiation risk of bleeding in patients irradiated for prostate cancer, Aliment. Pharmacol. Ther., 2011, vol. 34, no. 6, pp. 628–637.
Georges, G.E., Kuver, R.P., Jordan, R., et al., Post-exposure oral 17,21-beclomethasone dipropionate (BDP) improves survival in a canine gastrointestinal acute radiation syndrome (GI-ARS) model, in 58th Annual Meeting of the Radiation Research Society, San Juan, Lawrence, KS: Radiation Research Society, 2012.
Tarumov, R.A., Grebenyuk, A.N., and Basharin, V.A., Biological properties of the phytoestrogen genistein (review), Med. Ekstrem. Situats., 2014, no. 2, pp. 55–68.
Ganai, A.A. and Farooqi, H., Bioactivity of genistein: a review of in vitro and in vivo studies, Biomed. Pharmacother., 2015, vol. 76, pp. 30–38.
Weiss, J.F. and Landauer, M.R., Protection against ionizing radiation by antioxidant nutrients and phytochemicals, Toxicology, 2003, vol. 189, nos. 1–2, pp. 1–20.
Landauer, M.R., Srinivasan, V., and Seed, T.M., Genistein treatment protects mice from ionizing radiation injury, J. Appl. Toxicol., 2003, vol. 23, no. 6, pp. 379–385.
Zhou, Y. and Mi, M.T., Genistein stimulates hematopoiesis and increases survival in irradiated mice, J. Radiat. Res., 2005, vol. 46, no. 4, pp. 425–433.
Grace, M.B., Blakely, W.F., and Landauer, M.R., Genistein-induced alterations of radiation-responsive gene expression, Radiat. Meas., 2007, vol. 42, nos. 6–7, pp. 1152–1157.
Davis, T.A., Clarke, T.K., and Landauer, M.R., Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival, Int. J. Radiat. Biol., 2007, vol. 83, no. 3, pp. 141–151.
Davis, T.A., Mungunsukh, O., Zins, S., et al., Genistein induces radioprotection by hematopoietic stem cell quiescence, Int. J. Radiat. Biol., 2008, vol. 84, no. 9, pp. 713–726.
Grebenyuk, A.N., Tarumov, R.A., Basharin, V.A., et al., Experimental evaluation of the effect of synthetic genistein on hematologic indices and cytokine status of irradiated rats, Radiats. Biol. Radioecol., 2015, vol. 55, no. 2, pp. 160–168.
Grebenyuk, A.N., Tarumov, R.A., Basharin, V.A., and Kovtun, V.Yu., Experimental evaluation of radioprotective efficacy of synthetic genistein on criteria of glutathione system and lipid peroxidation in erythrocytes of peripheral blood in irradiated rats, Radiats. Biol. Radioecol., 2015, vol. 55, no. 5, pp. 501–506.
Ahmad, I.U., Forman, J.D., Sarkar, F.H., et al., Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer, Nutr. Cancer, 2010, vol. 62, no. 7, pp. 996–1000.
Tacyildiz, N., Ozyoruk, D., Yavuz, G., et al., Soy isoflavones ameliorate the adverse effects of chemotherapy in children, Nutr. Cancer, 2010, vol. 62, no. 7, pp. 1001–1005.
Yoon, S.I., Kurnasov, O., Natarajan, V., et al., Structural basis of TLR5-flagellin recognition and signaling, Science, 2012, vol. 335, no. 6070, pp. 859–864.
Burdelya, L.G., Brackett, C.M., Kojouharov, B., et al., Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist, Proc. Natl. Acad. Sci. U. S. A., 2013, vol. 110, no. 20, pp. E1857–E1866.
Burdelya, L.G., Krivokrysenko, V.I., Tallant, T.C., et al., An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models, Science, 2008, vol. 320, no. 5873, pp. 226–230.
Krivokrysenko, V.I., Toshkov, I.A., Gleiberman, A.S., et al., The Toll-like receptor 5 agonist entolimod mitigates lethal acute radiation syndrome in non-human primates, PLoS One, 2015, vol. 10, no. 9. e0135388. https://doi.org/10.1371/journal.pone.0135388
Krivokrysenko, V.I., Shakhov, A.N., Singh, V.K., et al., Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure, J. Pharmacol. Exp. Ther., 2012, vol. 343, no. 2, pp. 497–508.
Singh, V.K., Romaine, P.L., and Newman, V.L., Biologics as countermeasures for acute radiation syndrome: where are we now?, Expert. Opin. Biol. Ther., 2015, vol. 15, no. 4, pp. 465–471.
Ghosh, S.P., Perkins, M.W., Hieber, K., et al., Radiation protection by a new chemical entity, Ex-Rad: efficacy and mechanisms, Radiat. Res., 2009, vol. 171, no. 2, pp. 173–179.
Suman, S., Maniar, M., Fornace, A.J., and Datta, K., Administration of ON 01210.Na after exposure to ionizing radiation protects bone marrow cells by attenuating DNA damage response, Radiat. Oncol., 2012, vol. 7, p. 6. https://doi.org/10.1186/1748-717X-7-6
Suman, S., Datta, K., Doiron, K., et al., Radioprotective effects of ON 01210.Na upon oral administration, J. Radiat. Res., 2012, vol. 3, no. 3, pp. 368–376.
Ghosh, S.P., Kulkarni, S., Perkins, M.W., et al., Amelioration of radiation-induced hematopoietic and gastrointestinal damage by Ex-RAD® in mice, J. Radiat. Res., 2012, vol. 53, no. 4, pp. 526–536.
Kang, A.D., Cosenza, S.C., Bonagura, M., et al., ON01210.Na (Ex-RAD®) mitigates radiation damage through activation of the Akt pathway, PLoS One, 2013, vol. 8, no. 3. e58355. https://doi.org/10.1371/journal.pone.0058355
Grebenyuk, A.N., Strelova, O.Yu., Legeza, V.I., and Stepanova, E.N., Osnovy radiobiologii i radiatsionnoi meditsiny (Fundamentals of Radiobiology and Radiation Medicine), St. Petersburg: Foliant, 2012.
Legeza, V.I., Galeev, I.Sh., and Seleznev, A.B., Emeticheskii sindrom (Emetic Syndrome), St. Petersburg: Foliant, 2005.
Feyer, P.C., Maranzano, E., Molassiotis, A., et al., Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009, Supp. Care Cancer, 2011, vol. 19, suppl. 1, pp. 5–14.
Salvo, N., Doble, B., Khan, L., et al., Prophylaxis of radiation-induced nausea and vomiting using 5-hydroxytryptamine-3 serotonin receptor antagonists: a systematic review of randomized trials, Int. J. Radiat. Oncol. Biol. Phys., 2012, vol. 82, no. 1, pp. 408–417.
Legeza, V.I., Seleznev, A.B., and Drachev, I.S., Experimental evaluation of selective antagonists of serotonin 5HT3-receptors as means of prevention of the symptomatic complex of the primary response to irradiation in radiation accidents, Med.-Biol. Sots.-Psikhol. Probl. Bezop. Chrezv. Situats., 2011, no. 2, pp. 93–97.
Grebenyuk, A.N., Zatsepin, V.V., Nazarov, V.B., and Vlasenko, T.N., Modern opportunities of drug prevention and early treatment of radiation injuries, Voen.-Med. Zh., 2011, vol. 332, no. 2, pp. 13–17.
Khalimov, Yu.Sh., Grebenyuk, A.N., Karamullin, M.A., et al., Modern opportunities of providing therapeutic assistance in case of mass sanitary losses of radiation profile, Voen.-Med. Zh., 2012, vol. 333, no. 2, pp. 24–31.
Singh, V.K., Garcia, M., and Seed, T.M., A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part II. Countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status, Int. J. Radiat. Biol., 2017, vol. 93, no. 9, pp. 870–884.
Feyer, P., Seegenschmiedt, M.H., and Steingraeber, M., Granisetron in the control of radiotherapy-induced nausea and vomiting: a comparison with other antiemetic therapies, Support Care Cancer, 2005, vol. 13, no. 9, pp. 671–678.
Hsu, E.S., A review of granisetron, 5-hydroxytryptamine3 receptor antagonists, and other antiemetics, Am. J. Ther., 2010, vol. 17, no. 5, pp. 476–486.
Aleksakhin, R.M., Buldakov, L.A., Gubanov, V.A., et al., Krupnye radiatsionnye avarii: posledstviya i zashchitnye mery (Large-Scale Radiation Accidents: Consequences and Protective Measures), Il’in, L.A. and Gubanov, V.A., Eds., Moscow: IzdAT, 2001.
Grebenyuk, A.N. and Sidorov, D.A., Medical, social, and psychological aspects of radiological terrorism, Med.-Biol. Sots.-Psikhol. Probl. Bezop. Chrezv. Situats., 2012, no. 3, pp. 11–18.
Il’in, L.A., Radiological and nuclear terrorism—medical-biological and hygienic problems, Gig. Sanit., 2017, vol. 96, no. 9, pp. 809–812.
Rump, A., Becker, B., Eder, S., et al., Medical management of victims contaminated with radionuclides after a “dirty bomb” attack, Mil. Med. Res., 2018, vol. 5, p. 25. https://doi.org/10.1186/s40779-018-0174-5
Vasin, M.V., Sredstva profilaktiki i lecheniya luchevykh porazhenii (Means of Prevention and Treatment of Radiation Injuries), Moscow, 2006.
Krasnyuk, V.I. and Ustyugova, A.A., General principles of antidote therapy at radionuclide incorporation, Med. Truda. Prom. Ekol., 2017, no. 4, pp. 51–56.
Lyaginskaya, A.M., Ermalitskii, A.P., Osipov, V.A., et al., Provedenie iodnoi profilaktiki naseleniyu v sluchae vozniknoveniya radiatsionnoi avarii: metodicheskie rekomendatsii (Conducting Iodine Prophylaxis of Population in the Event of a Radiation Accident), Moscow: Federal’noe mediko-biologicheskoe agentstvo, 2010.
Iodine Thyroid Blocking: Guidelines for Use in Planning for and Responding to Radiological and Nuclear Emergencies, Geneva: World Health Organization, 2017.
Verger, P., Aurengo, A., Geoffroy, B., and Le Guen, B., Iodine kinetics and effectiveness of stable iodine prophylaxis after intake of radioactive iodine: a review, Thyroid, 2004, vol. 11, no. 4, pp. 353–360.
Zanzonico, P.B. and Becker, D.V., Effects of time of administration and dietary iodine levels on potassium iodide (KI) blockade of thyroid irradiation by 131I from radioactive fallout, Health Phys., 2000, vol. 78, pp. 660–667.
Guidance for Federal Agencies and State and Local Governments: Potassium Iodide Tablets Shelf Life Extension, FDA—Downloads—Drugs—Guidances, US Department of Health and Human Services, 2004. https://www.fda.gov/downloads/drugs/guidances/ ucm080549.pdf. Accessed October 18, 2018.
Kazzi, Z.N., Heyl, A., and Ruprecht, J., Calcium and zinc DTPA administration for internal contamination with plutonium-238 and americium-241, Curr. Pharm. Biotechnol., 2012, vol. 13, pp. 1957–1963.
Taylor, D.M., Stradling, G.N., and Hengé-Napoli, M.-H., The scientific background to decorporation, Radiat. Prot. Dosim., 2000, vol. 87, no. 1, pp. 11–18.
Zhang, Y., Sadgrove, M.P., Mumper, R.J., and Jay, M., Radionuclide decorporation: matching the biokinetics of actinides by transdermal delivery of pro-chelators, AAPS J., 2013, vol. 15, no. 4, pp. 1180–1188.
Stradling, G.N., Taylor, D.M., Hengé-Napoli, M.-H., et al., Treatment for actinide-bearing industrial dusts and aerosols, Radiat. Prot. Dosim., 2000, vol. 87, no. 1, pp. 41–50.
Wood, R., Sharp, C., Gourmelon, P., et al., Decorporation treatment-medical overview, Radiat. Prot. Dosim., 2000, vol. 87, no. 1, pp. 51–56.
Melo, D.R., Lipsztein, J.L., Leggett, R., et al., Efficacy of Prussian blue on 137Cs decorporation therapy, Health Phys., 2014, vol. 106, no. 5, pp. 592–597.
Perez, M. and Carr, Z., Report of the Radio-Nuclear Working Group.WHO Consultation Meeting on Development of Stockpiles for Radiation and Chemical Emergencies, Geneva: World Health Organization, 2007.
Altagracia-Martinez, M., Kravzov-Jinich, J., Martinez-Núnez, J.M., et al., Prussian blue as an antidote for radioactive thallium and cesium poisoning, Orphan Drugs: Res. Rev., 2012, vol. 2, pp. 13–21.
Rump, A., Stricklin, D., Lamkowski, A., et al., Reconsidering current decorporation strategies after incorporation of radionuclides, Health Phys., 2016, vol. 111, no. 2, pp. 204–211.
NIH Strategic Plan and Research Agenda for Medical Countermeasures Against Radiological and Nuclear Threats, NIH Publication no. 05-5608, U.S. Department of Health and Human Services, 2005.
Finashov, L.V. and Rafikov, U.M., Analysis of published data on prospective radioprotectants developed in the United States of America, Vopr. Radiats. Bezop., 2017, no. 2 (86), pp. 75–81.
Gudkov, S.V., Popova, N.R., and Bruskov, V.I., Radioprotective substances: history, trends and prospects, Biophysics (Moscow), 2015, vol. 60, no. 4, pp. 659–667.
Hofer, M., Hoferova, Z., Depes, D., and Falk, M., Combining pharmacological countermeasures to attenuate the acute radiation syndrome—a concise review, Molecules, 2017, vol. 22, no. 5. pii: E834. https://doi.org/10.3390/molecules22050834
Singh, V.K., Beattie, L.A., and Seed, T.M., Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures, J. Radiat. Res., 2013, vol. 54, no. 6, pp. 973–988.
Singh, P.K., Wise, S.Y., Ducey, E.J., et al., α-Tocopherol succinate protects mice against radiation-induced gastrointestinal injury, Radiat. Res., 2012, vol. 177, no. 2, pp. 133–145.
Singh, A. Thakkar, S., et al., Molecular dynamics guided design of tocof1exol: a new radioprotectant tocotrienol with enhanced bioavailability, Drug Dev. Res., 2014, vol. 75, no. 1, pp. 10–22.
Sridharan, V., Tripathi, P., Aykin-Burns, N., et al., Tocotrienol-enriched formulation protects against radiation-induced changes in cardiac mitochondria without modifying late cardiac function or structure, Radiat. Res., 2015, vol. 183, no. 3, pp. 357–366.
Li, X.H., Ha, C.T., Fu, D., et al., Delta-tocotrienol suppresses radiation-induced microRNA‑30 and protects mice and human CD34+ cells from radiation injury, PLoS One, 2015, vol. 10, no. 3. e0122258. https://doi.org/10.1371/journal.pone.0122258
Morita, A., Ariyasu, S., Sawa, A., Morita, A., et al., Design and synthesis of 8-hydroxyquinoline-based radioprotective agents, Bioorg. Med. Chem., 2014, vol. 22, no. 1, pp. 3891–3905.
Morita, A., Ariyasu, S., Wang, B., et al., AS-2, a novel inhibitor of p53-dependent apoptosis, prevents apoptotic mitochondrial dysfunction in a transcription-independent manner and protects mice from a lethal dose of ionizing radiation, Biochem. Biophys. Res. Commun., 2014, vol. 450, no. 4, pp. 1498–1504.
Chen, J.K., Li, Z.P., Liu, Y.Z., et al., Activation of alpha 7 nicotinic acetylcholine receptor protects mice from radiation-induced intestinal injury and mortality, Radiat. Res., 2014, vol. 181, no. 6, pp. 666–671.
Lee, C.L., Lento, W.E., Castle, K.D., et al., Inhibiting glycogen synthase kinase-3 mitigates the hematopoietic acute radiation syndrome in mice, Radiat. Res., 2014, vol. 181, no. 5, pp. 445–451.
Kim, J., Thimmulappa, R.K., Kumar, V., et al., NRF2-mediated notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation, J. Clin. Invest., 2014, vol. 124, no. 2, pp. 730–741.
Rowbottom, L., McDonald, R., Turner, A., et al., An overview of radiation-induced nausea and vomiting, J. Med. Imaging Radiat. Sci., 2016, vol. 47, no. 3, suppl., pp. S29–S38.
Singh, P., Yoon, S.S., and Kuo, B., Nausea: a review of pathophysiology and therapeutics, Ther. Adv. Gastroenterol., 2016, vol. 9, no. 1, pp. 98–112.
Drachev, I.S., Legeza, V.I., Seleznev, A.B., and Grebenyuk, A.N., On the nature of early disorders of neuroendocrine metabolism in animals irradiated with doses that are supralethal for rats, Radiats. Biol. Radioecol., 2018, vol. 58, no. 4, pp. 382–388.
Gladkikh, V.D. and Balandin, N.V., Opportunities of pharmacological correction of clinical manifestations of the primary response to irradiation, in Materialy XXIII s"ezda Fiziologicheskogo obshchestva im. I.P. Pavlova (Proc. XXIII Congr. Pavlov Physiol. Soc.), Voronezh: Istoki, 2017, pp. 830–833.
Kovtun, V.Yu., Davidovich, Yu.A., Proskurina, N.A., et al., Development of medicines for the prevention and relief of the primary response to irradiation, in Rossiskaya nauchnaya konferentriya “Mediko-biologicheskie problemy toksikologii i radiobiologii,” Tezisy dokladov (Russ. Sci. Conf. “Biomedical Problems of Toxicology and Radiobiology,” Abstracts of Papers), St. Petersburg, 2015, р. 148.
Drachev, I.S., Bykov, V.N., and Seleznev, A.B., Experimental study of the efficacy of palonosetron and phenotropil for preventing the primary response to irradiation, Radiats. Biol. Radioecol., 2016, vol. 56, no. 1, pp. 64–72.
An, D.D., Kullgren, B., Jarvis, E.E., and Abergel, R.J., From early prophylaxis to delayed treatment: establishing the plutonium decorporation activity window of hydroxypyridinonate chelating agents, Chem. Biol. Interact., 2017, vol. 267, pp. 80–88.
Zhang, Y., Sadgrove, M.P., Mumper, R.J., and Jay, M., Radionuclide decorporation: matching the biokinetics of actinides by transdermal delivery of pro-chelators, AAPS J., 2013, vol. 15, no. 4, pp. 1180–1188.
Huckle, J.E., Sadgrove, M.P., Pacyniak, E., et al., Orally administered DTPA diethyl ester for decorporation of 241Am in dogs: assessment of safety and efficacy in an inhalation-contamination model, Int. J. Radiat. Biol., 2015, vol. 91, no. 7, pp. 568–575.
Huckle, J.E., Sadgrove, M.P., and Leed, M.G., Synthesis and physicochemical characterization of a diethyl ester prodrug of DTPA and its investigation as an oral decorporation agent in rats, AAPS J., 2016, vol. 18, no. 4, pp. 972–980.
Grémy, O., Laurent, D., Coudert, S., et al., Decorporation of Pu/Am actinides by chelation therapy: new arguments in favor of an intracellular component of DTPA action, Radiat. Res., 2016, vol. 185, no. 6, pp. 568–579.
Gremy, O., Miccoli, L., Lelan, F., et al., Delivery of DTPA through liposomes as a good strategy for enhancing plutonium decorporation regardless of treatment regimen, Radiat. Res., 2018, vol. 189, no. 5, pp. 477–489.
Kovtun, V.Yu., Gladkikh, V.D., Davidovich, Yu.A., et al., On the use of dosage forms of pentacin and zincacin, Med. Radiol. Radiats. Bezop., 2015, vol. 60, no. 1, pp. 45–53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This the authors of this article did not perform studies involving animals or human participants.
Additional information
Translated by M. Novikova
Rights and permissions
About this article
Cite this article
Grebenyuk, A.N., Gladkikh, V.D. Modern Condition and Prospects for the Development of Medicines towards Prevention and Early Treatment of Radiation Damage. Biol Bull Russ Acad Sci 46, 1540–1555 (2019). https://doi.org/10.1134/S1062359019110141
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359019110141