Abstract—
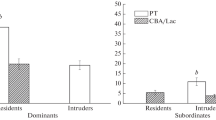
Chemocommunication plays an important role in establishing and maintaining the spatial ethological structure of the population. Previously, we demonstrated that the propensity to social dominance in male laboratory mice is mainly determined by an individual’s genotype. However, it remains unclear whether genotypic peculiarities of mouse olfactory communication, the most important links of which are marking behavior and olfactory contacts, are associated with the ability to occupy a high social rank in the hierarchical structure of the community? The aim of the present work was to establish the effect of genotype on the intensity of marking behavior and olfactory contacts in male laboratory mice in a social competition model and to study the association of these types of social behavior with a hereditary predisposition to dominance. This study was conducted on adult males of inbred mice strains BALB/cLac, CBA/Lac, and PT differing in the propensity to dominate: BALB/cLac and PT males mainly dominated CBA/Lac males. The experimental groups were formed out of two males of different genotypes in three possible pairwise combinations. The intensity of urine marking the territory was estimated for each male in conditions of social isolation and after pair keeping during the period of stable dominant–subordinate relations. Olfactory contacts that were naso-nasal and naso-anogenital sniffing were used as an additional indicator of the propensity of mice to olfactory communication in conditions of social competition. Hereditary differences in the intensity of urine marking and in the frequency of olfactory contacts were established in male laboratory mice in the social competition model: dominant males of the CBA/Lac strain marked the territory and sniffed the opponent more frequently than dominants of the BALB/cLac and PT strains, while a decrease in urine marking and olfactory activity was observed in subordinate males of these strains. During social isolation, males of the CBA/Lac strain also marked the territory more frequently than males of the two other strains. Thus, the male mice of different inbred strains differ in the intensity of urine marking and olfactory behavior, which can be modified by external conditions, for example, social rank of an individual, and the intensity of urine marking and olfactory activity does not predict the social rank of an individual.


Similar content being viewed by others
REFERENCES
An, X.L., Zou, J.X., Wu, R.Y., Yang, Y., Tai, F.D., et al., Strain and sex differences anxiety-like and social behaviors of C57BL/6J and BALB/cJ mice, Exp. Anim., 2011, vol. 60, pp. 111–123.
Arakawa, H., Blanchard, D.C., Arakawa, K., Dunlapc, C., and Blanchard, R.J., Scent marking behavior as an odorant communication in mice, Neurosci. Biobehav. Rev., 2008, vol. 32, pp. 1236–1248.
Beauchamp, G.K. and Yamazaki, K., Chemical signalling in mice, Biochem. Soc. Trans., 2003, vol. 31, pt. 1, pp. 147–151.
Bolivar, V.J., Walters, S.R., and Phoenix, J.L., Assessing autism-like behavior in mice: variations in social interactions among inbred strains, Behav. Brain Res., 2007, vol. 176, pp. 21–26.
Borysik, A.J., Briand, L., Taylor, A.J., and Scott, D.J., Rapid odorant release in mammalian odour binding proteins facilitates their temporal coupling to odorant signals, J. Mol. Biol., 2010, vol. 404, pp. 372–380.
Bragin, A.V., Osadchuk, L.V., and Osadchuk, A.V., Experimental model of the formation and maintenance of social hierarchy in laboratory mice, Zh. Vyssh. Nerv. Deyat., 2006, vol. 56, no. 3, pp. 412–419.
Brennan, P.A. and Kendrick, K.M., Mammalian social odours: attraction and individual recognition, Philos. Trans. R. Soc., B., 2006, vol. 361, pp. 2061–2078.
Desjardins, C., Maruniak, J.A., and Bronson, F.H., Social rank in house mice: differentiation revealed by ultraviolet of urinary marking patterns, Nature, 1973, vol. 20, pp. 939–941.
Drickamer, L.C., Urine marking and social dominance in male house mice (Mus musculus domesticus), Behav. Proc., 2001, vol. 53, pp. 113–120.
Fairless, A.H., Katz, J.M., Vijayvargiya, N., Dow, H.C., Kreibich, A.S., et al., Development of home cage social behaviors in BALB/cJ vs. C57BL/6J mice, Behav. Brain Res., 2013, vol. 237, pp. 338–347.
Gromov, V.S., Prostranstvenno-etologicheskaya struktura populyatsii gryzunov (Spatial-Ethological Structure of Rodent Populations), Moscow: Tov. Nauchn. Izd. KMK, 2008.
Gromov, V.S., Scent marking in gerbils and its possible functions, Russ. J. Theriol., 2015, vol. 14, no. 1, pp. 113–126.
Huchard, E., English, S., Bell, M.B., Thavarajah, N., and Clutton-Brock, T., Competitive growth in a cooperative mammal, Nature, 2016, vol. 533, no. 7604, pp. 532–534.
Hurst, J.L., Urine marking in populations of wild house mice Mus domesticus rutty. I. Communication between males, Anim. Behav., 1990, vol. 40, pp. 209–222.
Hurst, J.L., Female recognition and assessment of males through scent, Behav. Brain Res., 2009, vol. 200, pp. 295–303.
Hurst, J.L. and Beynon, R.J., Scent wars: the chemobiology of competitive signaling in mice, BioEssays, 2004, vol. 26, pp. 1288–1298.
Hurst, J.L., Robertson, D.H.L., Tolladay, U., and Beynon, R.J., Proteins in urine scent marks of male house mice extend the longevity of olfactory signals, Anim. Behav., 1998, vol. 55, pp. 1289–1297.
Kelliher, K.R. and Wersinger, S.R., Olfactory regulation of the sexual behavior and reproductive physiology of the laboratory mouse: effects and neural mechanisms, ILAR J., 2009, vol. 50, pp. 28–42.
Kepecs, A., Uchida, N., and Mainen, Z.F., The sniff as a unit of olfactory processing, Chem. Sens., 2006, vol. 31, pp. 167–179.
Kleshchev, M.A. and Osadchuk, L.V., Age dynamics of olfactory contacts in male laboratory mice in a social competition: the role of genotype, Ekol. Genet., 2013, vol. 11, no. 1, pp. 36–41.
Koyama, S., Primer effects by conspecific odors in house mice: a new perspective in the study of primer effects on reproductive activities, Horm. Behav., 2004, vol. 46, pp. 303–310.
Liberles, S.D., Mammalian pheromones, Ann. Rev. Physiol., 2014, vol. 76, pp. 151–175.
Maslak, S. and Gouat, P., Short-term contact elicits heterospecific behavioral discrimination of individual odors in mound-building mice (Mus spicilegus), J. Comp. Psychol., 2002, vol. 116, pp. 357–362.
Nelson, A.C., Cunningham, C.B., Ruff, J.S., and Potts, W.K., Protein pheromone expression levels predict and respond to the formation of social dominance networks, J. Evol. Biol., 2015, vol. 28, pp. 1213–1224.
Novikov, S.N., Coordinated expression of the genes Gus and Mup as a potential basis of the functional activity of the androgen-dependent pheromones of the house mouse (Mus musculus L.), Dokl. Biol. Sci., 2003, vol. 391, no. 5, pp. 318–321.
Novikov, S.N., Churakov, G.A., Filimonenko, A.A., Ermakova, I.I., Fedorova, E.M., and Burkot, I.A., . The pattern of major urinary proteins (MUPs) expression during postnatal ontogenesis of the laboratory mouse depends on genotype and sex, Russ. J. Dev. Biol., 2009, vol. 40, no. 4, pp. 204–211.
Osadchuk, L.V., Testicular function in mice of inbred strains BALB/cLac, PT, and CBA/Lac, Ross. Fiziol. Zh. im. I.M. Sechenova, 2010, vol. 96, no. 2, pp. 183–190.
Osadchuk, L.V., Parameters of spermatogenesis and testosterone production during sexual maturation as predictors of the functional activity of the testes in laboratory mice (Mus musculus), Zh. Evol. Biokhim. Fiziol., 2016, vol. 52, no. 6, pp. 423–428.
Osadchuk, L.V., Bragin, A.V., and Osadchuk, A.V., Interstrain differences in social and time patterns of agonistic behavior in male laboratory mice, Zh. Vyssh. Nerv. Deyat.im. I.P. Pavlova, 2009, vol. 59, no. 4, pp. 473–481.
Sankoorikal, G.M., Kaercher, K.A., Boon, C.J., Lee, J.K., and Brodkin, E.S., A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains, Biol. Psychiatry, 2006, vol. 59, pp. 415–423.
Sokolov, V.E., Zagoruiko, N.V., Kotenkova, E.V., and Lyalyukhina, S.I., Comparative analysis of the behavior of house (Mus musculus musculus) and immunodeficient (Mus hortulanus) mice, Zool. Zh., 1988, vol. 67, no. 8, pp. 1214–1224.
Surov, A.V. and Mal’tsev, A.N., Analysis of chemical communication in mammals: zoological and ecological aspects, Biol. Bull. (Moscow), 2016, vol. 43, no. 9, pp. 1175–1183.
Thom, M.D. and Hurst, J.L., Individual recognition by scent, Ann. Zool. Fenn., 2004, vol. 41, pp. 765–787.
Wesson, D.W., Varga-Wesson, A.G., Borkowski, A.H., and Wilson, D.A., Respiratory and sniffing behaviors throughout adulthood and aging in mice, Behav. Brain Res., 2011, vol. 223, pp. 99–106.
Funding
This work was performed within the state task “Cellular and Molecular Genetic Mechanisms of the Control of Adaptive and Pathological Processes in Humans and Animals” no. 0324-2018-0016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement of the welfare of animals. All experimental procedures were performed according to the rules of scientific research using experimental animals approved by the order of the Presidium of USSR Academy of Sciences from April 2, 1980, no. 12000-496 and by the order of USSR Ministry of Higher Education from September 13, 1984, no. 22.
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Osadchuk, L.V., Osadchuk, A.V. Genotypic Peculiarities of Olfactory Communication in Male Laboratory Mice (Mus musculus) in a Social Competition Model. Biol Bull Russ Acad Sci 46, 966–972 (2019). https://doi.org/10.1134/S1062359019080120
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359019080120