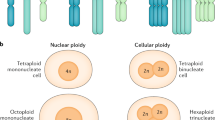
Abstract
In this study, we used the mouse model of chemically induced hepatocarcinogenesis to investigate the chromosomal aberrations in hepatic cells. The model was obtained by combined treatment of mice with Dipin (radiomimetic drug) followed by partial hepatectomy. Cytological analysis of isolated liver cells treated with Dipin has demonstrated a number of hepatocytes with structural nuclear abnormalities and multiple micronuclei. Karyotype analysis of polyploid hepatocytes has shown numerous chromosomal aberrations including alleged morphological manifestations of chromothripsis, a special type of genomic reorganization characterized by the local disintegration of chromosomes. Micronuclei with chromosomal fragments have developed as a result of double-strand DNA breaks and might serve as the initial substrate for chromothripsis. The emergence of micronuclei containing chromosomal fragments is the most important result of the treatment employed. Therefore, the presented model of liver cancer (hepatocarcinogenesis) can be used to study the process of chromothripsis in the future.
Similar content being viewed by others
References
Belov, L.N., Kogan, M.E., Leont’eva, T.A., Kostyrev, O.A., and Tsellarius, Yu.G., Preparation of isolated cells by alkaline dissociation of formalin-fixed tissues, Tsitologiia, 1975, vol. 17, pp. 1332–1337.
Branzey, D. and Foliani, M., Regulation of DNA repair throughout the cell cycle, Nat. Rev. Mol. Cell Biol., 2008, vol. 9, pp. 297–308.
Crasta, K., Ganem, N.J., Dagher, R., Lantermann, A.B., Ivanova, E.V., Pan, Y.P., Nezi, L., Protopopov, A., Choudry, D., and Pellman, D., DNA breaks and chromosome pulverization from errors in mitosis, Nature, 2012, vol. 482, pp. 53–58.
Faktor, V.M., Uryvaeva, I.V., Sokolova, A.S., Chernov, V.A., and Brodsky, W.Ya., Kinetics of cellular proliferation in regenerated mouse liver pretreated with the alkylating drug dipin, Virchows Archiv. B: Cell Path., 1980, vol. 33, pp. 187–197.
Faktor, V.M., Eliseeva, N.A., and Tamakhina, A.Ya., Effect of the alkylating carcinogen dipin on proliferation, polyploidy development level, and formation of micronuclei in the population of the initial and de novo formed hepatocytes, Izv. Akad. Nauk, Ser. Biol., 1992, vol. 6, pp. 821–834.
Factor, V.M., Laskowska, D., Jensen, M.R., Woitach, J.T., Popescu, N.C., and Thorgeirsson, S.S., Vitamin E reduces chromosomal damage and inhibits hepatic tumor formation in a transgenic mouse model, Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, pp. 2196–2201.
Fenech, M., Kirsch-Folders, M., Natarajan, A.T., Surralles, J., Crott, J.W., Parry, J., Norppa, H., Eastmond, D.A., Tucker, J.D., and Thomas, P., Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells, Mutagenesis, 2011, vol. 26, pp. 125–132.
Fukami, M., Shima, H., Suzuki, E., Ogata, T., Matsubara, K., and Kamimaki, T., Catastrophic cellular events leading to complex chromosomal rearrangements in the germline, Clin. Genet., 2017, vol. 91, pp. 653–660.
Hatch, E.M., Y chromothripsis, Nat. Cell Biol., 2017, vol. 19, pp. 12–14.
Heng, H.H.Q., Liu, G., Stevens, J.B., Abdallah, B.Y., Horne, S.D., Ye, K.L., Bremer, S.W., Chowdhury, S.K., and Ye, C.J., Karyotype heterogeneity and unclassified chromosomal abnormalities, Cytogenet. Genome Res., 2013, vol. 139, pp. 144–157.
Hintzsche, H., Hemann, U., Poth, A., Utesch, D., Lott, J., and Stopper, H., Fate of micronuclei and micronucleated cells, Mutat. Res., 2017, vol. 771, pp. 85–98.
Ikeuchi, T., Weinfeld, H., and Sandberg, A.A., Chromosome pulverization in micronuclei induced by tritiated thymidine, J. Cell Biol., 1972, vol. 52, pp. 97–104.
Ivkov, R. and Bunz, F., Pathways to chromothripsis, Cell Cycle, 2015, vol. 14, pp. 2886–2890.
Jensen, M.R., Factor, V.M., and Thorgeirsson, S.S., Regulation of cyclin G1 during murine hepatic regeneration following dipin-induced DNA damage, Hepatology, 1998, vol. 28, pp. 537–546.
Johnson, R.T. and Rao, P.N., Mammalian cell fusion: induction of premature chromosome condensation in interphase nuclei, Nature, 1970, vol. 226, no. 5247, pp. 717–722.
Kloosterman, W.P. and Cuppen, E., Chromothripsis in congenital disorders and cancer: similarities and differences, Curr. Opin. Cell Biol., 2013, vol. 25, pp. 341–348.
Liu, G., Stevens, J.B., Horne, S.D., Abdallah, B.Y., Ye, K.J., Bremer, S.W., Ye, C.J., Chen, D.J., and Heng, H.H., Genome chaos: survival strategy during crisis, Cell Cycle, 2014, vol. 13, pp. 528–537.
Ly, P. and Cleveland, D.W., Rebuilding chromosomes after catastrophe: emerging mechanisms of chromothripsis, Trends Cell Biol., 2017, vol. 27, pp. 917–930.
Ly, P., Teitz, L.S., Kim, D.H., Shoshany, O., Skaletsky, H., Fatinetti, D., Page, D.C., and Cleveland, D.W., Selective Y centromere inactivation triggers chromosome shattering in micronuclei and repair by non-homologous end joining, Nat. Cell Biol., 2017, vol. 19, pp. 68–75.
MacKinnon, R.N. and Campbell, L.J., Chromothripsis under the microscope; a cytogenetic perspectives of two cases of AML with catastrophic chromosome rearrangement, Cancer Genet., 2013, vol. 206, pp. 238–251.
Mamaev, N.N., Gindina, T.L., and Boichenko, E.G., Chromothripsis in oncology: a review of literature and own observation, Klinich. Onkogematol., 2017, vol. 10, pp. 191–205.
Mardin, B.R., Drainas, A.P., Waszak, S.M., Weischenfeldt, J., Isokane, M., Stütz, A.M., Raeder, B., Efthymiopoulos, T., Buccitelli, C., Segura-Wand, M., Northcott, P., Pfister, S.M., Ellenberg, J., Lichter, P., and Korbel, J.O., A cell-based model system links chromothripsis with hyperploidy, Mol. Syst. Biol., 2015, vol. 11, pp. 828–840.
McDermott, D., Gao, J.L., Liu, Q., Siwicki, M., Martens, C., Jacobs, P., Velez, D., Yim, E., Bryke, C.R., Hsu, N., Dai, Z., Marquesen, M.M., Stregevski, E., Kwatemaa, N., Theobald, N., Long Priel Da, Pittaluga, S., Raffeld, M.A., Calvo, K.R., Maric, I., Desmond, R., Holmes, K.L., Kuhns, D.B., Balabanian, K., Bachelerie, F., Porcella, S.F., Malech, H.L., and Murphy, P.M., Chromothripsis cure of WHIM syndrome, Cell, 2015, vol. 160, pp. 686–699.
Meyerson, M. and Pellman, D., Cancer genomes evolve by pulverizing single chromosomes, Cell, 2011, vol. 144, pp. 9–10.
Morishita, M., Muramatsu, T., Suto, Y., Hirai, M., Konishi, T., Hayashi, Sh., Shigemisu, D., Tsunoda, T., Moriyama, K., and Inazava, J., Chromotripsis-like chromosomal rearrangements induced by ionizing radiation using proton microbeam irradiation system, Oncotarget, 2016, vol. 7, pp. 1082–1092.
Nowell, P.C., The clonal evolution of tumor cell populations, Science, 1976, vol. 194, pp. 23–28.
Pellestor, F., Gatinois, V., Puechberty, J., Genevieve, D., and Lefort, G., Chromothripsis: potential origin in gametogenesis and preimplantation cell division. A review, Fertil. Steril., 2014, vol. 102, pp. 1785–1796.
Poot, M., Of simple and complex genome rearrangements, chromothripsis, chromoanasynthesis, and chromosome chaos, Mol. Syndromol., 2017, vol. 8, pp. 115–117.
Rode, A., Maas, K., Willmund, K., Lichter, P., and Ernst, A., Chromothripsis in cancer cells: an update, Int. J. Cancer, 2015, vol. 138, pp. 2322–2333.
Sargent, L.M., Sanderson, N.D., and Thorgeirsson, S.S., Ploidy and karyotypic alteration associated with early events in the development of hepatocarcinogenesis in transgenic mice harboring c-myc and transforming growth factor α transgenes, Cancer Res., 1996, vol. 56, pp. 2137–2142.
Skuija, E., Kalniete, D., Nagazawa-Miklasevica, M., Daneberga, Z., Abolins, A., Purkalne, G., and Miklasevics, E., Chromothripsis and progression-free survival in metastatic colorectal cancer, Mol. Clinic. Oncol., 2017, vol. 6, pp. 182–186.
Stephens, P.J., Greeman, C.D., Fu, B., Yang, F., Bignell, G.R., Mudie, L.J., Pleasans, E.D., Lau, K.W., Beare, D., Stebbings, L.A., McLaren, S., Lin, M.L., McBride, D.J., Varela, I., Nik-Zainal, S., Leroy, C., Jia, M., Menzies, A., Butler, A.P., Teague, J.W., Quali, M.A., Burton, J., Swerdlow, H., Carter, N.P., Morsberger, L.A., Iacobusio-Donahue, Ch., Follows, G.A., Green, A.R., Flanagan, A.M., Stratton, M.R., Futreal, P.A., and Campbell, P.J., Massive genome rearrangement acquired in a single catastrophic event during cancer development, Cell, 2011, vol. 144, pp. 27–40.
Uryvaeva, I.V., A model of hepatic regeneration and carcinogenesis due to total liver cell injury induced by dipin and partial hepatectomy, Monogr. Dev. Biol., Basel: Karger, 1992, vol. 23, pp. 230–236.
Uryvaeva, I.V. and Delone, G.V., The assessment of the level of genetic damages accumulated with age and induced in liver cells by the micronucleus production, Ontogenez, 1992, vol. 23, pp. 370–377.
Uryvaeva, I.V. and Delone, G.V., An improved method of liver micronucleus analysis: an application to age-related genetic alteration and polyploidy study, Mutat. Res., 1995, vol. 334, pp. 71–80.
Uryvaeva, I.V., Delone, G.V., and Marshak, T.L., Development of nuclear anomalies and cytomegalic degeneration as a result of genome lesion in hepatocytes, Dokl. Biol. Sci., 1996a, vol. 348, pp. 317–320.
Uryvaeva, I.V., Marshak, T.L., and Delone, G.V., Cell cycle during survival of liver cells after potentially lethal damage of genome induced by dipin, Bull. Eksp. Biol. Med., 1996b, vol. 122, pp. 353–355.
Zhang, Ch.-Zh., Spector A., Cornils H., Francis J.V., Jackson E.K., Liu, Sh., Meyerson, M., and Pellman, D., Chromotripsis from DNA damage in micronuclei, Nature, 2015, vol. 522, pp. 179–184.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.V. Uryvaeva, A.S. Mikaelyan, N.O. Dashenkova, T.L. Marshak, 2018, published in Izvestiya Akademii Nauk, Seriya Biologicheskaya, 2018, No. 5, pp. 461–468.
Rights and permissions
About this article
Cite this article
Uryvaeva, I.V., Mikaelyan, A.S., Dashenkova, N.O. et al. Chromothripsis in Hepatocarcinogenesis: The Role of a Micronuclear Aberration and Polyploidy. Biol Bull Russ Acad Sci 45, 419–425 (2018). https://doi.org/10.1134/S1062359018050163
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359018050163