Abstract
Stress reactions with activation of the sympathetic-adrenal system due to acute hypoxia reflects the degree of sensitivity of the body to this extreme factor. Succinate dehydrogenase (SDH) activation in cells as an adaptive response to acute hypoxia is closely associated with the degree of disturbance of tissue respiration through a lack of oxygen in the tissues, including the manifestation of “biochemical shock,” which is an unavoidable component of implementation of the protective effect of radioprotectors. In experiments on mice, rats, and dogs, the correlation between the manifestation of the radioprotective effect of acute hypoxia and SDH activation in blood lymphocytes, caused primarily by adrenergic stimulation during stress reactions, is confirmed. The degree of SDH activation in blood lymphocytes by hypoxia of different origins including that induced by radioprotectors may indicate its radioprotective potential irrespective of the differences in the oxygen consumption intensity and the resistance to acute hypoxia in animals and humans.
Similar content being viewed by others
References
Akopova, O., Nosar, V., and Gavenauskas, B., The effect of ATP-dependent potassium uptake on mitochondrial functions under acute hypoxia, J. Bioenerg. Biomembr., 2016, vol. 48, no. 1, pp. 67–75.
Allalunis-Turner, M.J., Reduced bone marrow pO2 following treatment with radioprotective drugs, Radiat. Res., 1990, vol. 122, no. 3, pp. 262–267.
Allalunis-Turner, M.J., Walden, T.J., and Sawich, C., Induction of marrow hypoxia by radioprotective agents, Radiat. Res., 1989, vol. 118, no. 3, pp. 581–586.
Antipov, V.V., Vasin, M.V., and Gaidamakin, A.N., Species-specific reactions of succinate dehydrogenase of lym- phocytes in animals to acute hypoxic hypoxia and its relation to the radiation resistance of the body, Kosm. Biol. Aviakosm. Med., 1989, vol. 23, no. 2, pp. 63–66.
Ariza, A.C., Deen, P.M., and Robben, J.H., The succinate receptor as a novel therapeutic target for oxidative and metabolic stress-related conditions, Front. Endocrinol. (Lausanne), 2012, vol. 3, p. 22.
Bacq, Z.M., Beaumariage, M.L., and Van Caneghem, P., Importance for radioprotective effect in mammals of pharmacological and biochemical actions of cysteamine and related substances, Ann. Ist. Super Sanita, 1965, vol. 1, no. 9, pp. 639–645.
Bacq, Z.M., Beaumariage, M.L., Goutier, R., and Van Caneghem, P., The state of shock induced by cystamine and cysteamine, Br. J. Pharmacol., 1968, vol. 34, no. 1, p. 202–203.
Cavanagh, H.D. and Colley, A.M., Cholinergic, adrenergic, and PGE1 effects on cyclic nucleotides and growth in cultured corneal epithelium, Metab. Pediatr. Syst. Ophthalmol., 1982, vol. 6, no. 2, pp. 63–74.
Dawson, T.L., Gores, G.J., Nieminen, A.L., Herman, B., and Lemasters, J.J., Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes, Am. J. Physiol. Cell Physiol., 1993, vol. 264, no. 4, pt 1, pp. C961–C967.
Duong, T.T., Witting, P.K., Antao, S.T., Parry, S.N., Kennerson, M., Lai, B., Vogt, S., Lay, P.A., and Harris, H.H., Multiple protective activities of neuroglobin in cultured neuronal cells exposed to hypoxia re-oxygenation injury, J. Neurochem., 2009, vol. 108, no. 5, pp. 1143–1154.
Feldkamp, T., Kribben, A., Roeser, N.F., Senter, R.A., Kemner, S., Venkatachalam, M.A., Nissim, I., and Weinberg, J.M., Preservation of complex I function during hypoxia-reoxygenation-induced mitochondrial injury in proximal tubules, Am. J. Physiol. Renal. Physiol., 2004, vol. 286, no. 4, pp. F749–F759.
Fernandez-Gomez, F.J., Galindo, M.F., Gómez-Lázaro, M., Yuste, V.J., Camello, J.X., Aguirre, N., and Jordan, J., Malonate induces cell death via mitochondrial potential collapse and delayed swelling through an ROS-dependent pathway, Br. J. Pharmacol., 2005, vol. 144, no. 4, pp. 528–537.
Firket, H. and Beaumariage, M.L., Ultrastructural modifications of mitochondria and rough endoplasmic reticulum of liver and spleen after a period of hypoxia, Virchows Arch. Cell Pathol., 1971, vol. 8, no. 4, pp. 342–349.
Firket, H. and Lelièvre, P., Effect of cystamine on the respiration, oxidative phosphorylation and ultrastructure of the mitochondria of the rat, Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 1966, vol. 10, no. 4, pp. 403–415.
Fujishiro, N., Endo, Y., Warashina, A., and Inoue, M., Mechanisms for hypoxia detection in O2-sensitive cells, Jpn. J. Physiol., 2004, vol. 54, no. 2, pp. 109–123.
Gaidamakin, A.N. and Abramov, M.M., Comparison of changes in succinate dehydrogenase activity in blood lymphocytes and modification of radiosensitivity by exogenous hypoxia, Radiobiologiya, 1987, vol. 27, no. 4, pp. 524–528.
Garlid, K.D. and Paucek, P., The mitochondrial potassium cycle, IUBMB Life, 2001, vol. 52, pp. 153–158.
Haass, M., Richardt, G., and Schömig, A., Potentiation of potassium-evoked noradrenaline and neuropeptide Y corelease by cardiac energy depletion: role of calcium channels and sodium-proton exchange, Naunyn Schmiedebergs Arch. Pharmacol., 1992, vol. 346, no. 4, pp. 410–418.
Hakak, Y., Lehmann-Bruinsma, K., Phillips, S., Le, T., Liaw, C., Connolly, D.T., and Behan, D.P., The role of the GPR91 ligand succinate in hematopoiesis, J. Leukocyte Biol., 2009, vol. 85, no. 5, pp. 837–843.
Hasegawa, A.T. and Landahl, H.D., Studies on spleen oxygen tension and radioprotection in mice with hypoxia, serotonin and p-aminopropiophenone, Radiat. Res., 1967, vol. 31, no. 3, pp. 389–399.
Hawkins, B.J., Levin, M.D., Doonan, P.J., Petrenko, N.B., Davis, C.W., Patel, V.V., and Madesh, M., Mitochondrial complex IIprevents hypoxic but not calcium- and proapoptotic Bcl-2 protein-induced mitochondrial membrane potential loss, J. Biol. Chem., 2010, vol. 285, no. 34, pp. 26494–26505.
He, W., Miao, F.J., Lin, D.C., Schwandner, R.T., Wang, Z., Gao, J., Chen, J.L., Tian, H., and Ling, L., Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors, Nature, 2004, vol. 429, no. 6988, pp. 188–193.
Hu, J., Bernardini, A., and Fandrey, J., Optical analysis of hypoxia inducible factor (HIF)-1 complex assembly: imaging of cellular oxygen sensing, Adv. Exp. Med. Biol., 2016, vol. 903, pp. 247–258.
Jones, D.P. and Mason, H.S., Gradients of O2 concentration in hepatocytes, J. Biol. Chem., 1978, vol. 253, no. 14, pp. 4874–4880.
Kaasik, A., Safiulina, D., Zharkovsky, A., and Veksler, V., Regulation of mitochondrial matrix volume, Am. J. Physiol. Cell Physiol., 2007, vol. 292, no. 1, pp. C157–C163.
Kawada, T., Yamazaki, T., Akiyama, T., Sato, T., Shishido, T., Inagaki, M., Tatewaki, T., Yanagiya, Y., Sugimachi, M., and Sunagawa, K., Cyanide intoxication induced exocytotic epinephrine release in rabbit myocardium, J. Auton. Nerv. Syst., 2000, vol. 80, no. 3, pp. 137–141.
Kehrer, J.P. and Lund, L.G., Cellular reducing equivalents and oxidative stress, Free Radic. Biol. Med., 1994, vol. 17, no. 1, pp. 65–75.
Kondrasheva, M.N., Activation of succinate dehydrogenase as the basis for anaerobic performance and resistance to hypoxia, in Mitokhondrial’nye protsessy vo vremennoi organizatsii zhiznedeyatel’nosti (Mitochondrial Processes in the Temporal Organization of Life Activity), Kondrasheva, M.N. and Maevskii, E.I., Eds., Pushchino, 1978, pp. 6–12.
Kondrasheva, M.N. and Chagovets, N.R., Succinic acid in the skeletal muscles during intense load and relaxation, Dokl. Akad. Nauk SSSR, 1971, vol. 198, no. 1, pp. 243–246.
Kondrasheva, M.N., Maevskii, E.I., Babayan, E.I., and Akhmerov, R.N., Adaptation to hypoxia by switching metabolism to the conversion of succinic acid, in Mitokhondriya. Biokhimiya i ul’trastruktura (Mitochondrion: Biochemistry and Ultrastructure), Moscow: Nauka, 1973, pp. 112–129.
Kondrashova, M., Zakharchenko, M., and Khunderyakova, N., Preservation of the in vivo state of mitochondrial network for ex vivo physiological study of mitochondria, Int. J. Biochem. Cell Biol., 2009, vol. 41, no. 10, pp. 2036–2050.
Konstantinova, M.M. and Graevskii, E.I., Tissue hypoxia as a mechanism of radioprotective action of adrenaline, heroin, and morphine, Dokl. Akad. Nauk SSSR, 1960, vol. 133, pp. 1427–1430.
Koroleva, L.V. and Vasin, M.V., Effect of adrenaline on the succinate dehydrogenase activity of peripheral blood lymphocytes of rats following exposure to ionizing radiation, Radiobiologiya, 1988, vol. 28, no. 2, pp. 228–230.
Kraus-Friedmann, N., Glucagon-stimulated respiration and intracellular Ca2+, FEBS Lett., 1986, vol. 201, no. 1, pp. 133–136.
Kulinskii, V.I., Kuntsevich, A.K., and Trufanova, L.V., Activation succinate dehydrogenation in rat liver by noradrenaline, cAMP and acute cooling, Byul. Eksp. Biol. Med., 1981, vol. 92, no. 8, pp. 33–34.
Kurz, T., Richardt, G., Seyfarth, M., and Schömig, A., Nonexocytotic noradrenaline release induced by pharmacological agents or anoxia in human cardiac tissue, Naunyn Schmiedebergs Arch. Pharmacol., 1996, vol. 354, no. 1, pp. 7–16.
Kvetnansky, R., Lu, X., and Ziegler, M.G., Stress-triggered changes in peripheral catecholaminergic systems, Adv. Pharmacol., 2013, vol. 68, pp. 359–397.
Laszczyca, P., The activity of mitochondrial enzymes in the muscles of rats subjected to physical training and subchronical intoxication with lead and zinc, Acta Physiol. Pol., 1989, vol. 40, nos. 5–6, pp. 544–551.
Lehninger, A.L. and Remmert, L.F., An endogenous uncoupling and swelling agent in liver mitochondria and its enzymic formation, J. Biol. Chem., 1959, vol. 234, pp. 2459–2464.
Lehninger, A.L. and Schneider, M., Mitochondrial swelling induced by glutathione, J. Biophys. Biochem. Cytol., 1959, vol. 5, no. 1, pp. 109–116.
Lelièvre, P., Action of cystamine and cysteamine on the oxygen consumption and coupled oxidative phosphorylation of the liver mitochondria of rats, Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 1965, vol. 9, pp. 107–113.
Luk’yanova, L.D., Mitochondrial signaling pathways in adaptation to hypoxia, Fiziol. Zh. im. I.M. Sechenova, 2013, vol. 59, no. 6, pp. 141–154.
Maevskii, E.I., Grishina, E.V., Rozenfel’d, A.S., Zyakun, A.M., Kondrashova, M.N., and Vereshchagina, V.M., Anaerobic conversion of succinate and enhancement of its oxidation. Possible mechanisms of cellular adaptation to oxygen deficiency, Biofizika, 2000, vol. 45, no. 3, pp. 509–513.
Van der Meer, C. and van Bekkum, D.W., The mechanism of radiation protection by hystamine and other biological amines, Int. J. Radiat. Biol., 1959, vol. 1, pp. 5–12.
Van der Meer, C. and van Bekkum, D., A study on the mechanism of radiation protection by 5-hydroxytryptamine and tryptamine, Int. J. Radiat. Biol., 1961, vol. 4, pp. 105–110.
Mohan, C., Memon, R.A., and Bessman, S.P., Differential effects of insulin, epinephrine, and glucagon on rat hepatocyte mitochondrial activity, Arch. Biochem. Biophys., 1991, vol. 287, no. 1, pp. 18–23.
Mojet, M.H., Mills, E., and Duchen, M.R., Hypoxiainduced catecholamine secretion in isolated newborn rat adrenal chromaffin cells is mimicked by inhibition of mitochondrial respiration, J. Physiol., 1997, vol. 504, no. 1, pp. 175–189.
Nartsissov, R.P., The use of N-nitrotetrazolium violet for quantitative cytometry of dehydrogenases in human lymphocytes, Arkh. Anat. Gistol. Embriol., 1969, vol. 56, no. 5, pp. 85–91.
Newmeyer, D.D. and Ferguson-Miller, S., Mitochondria: releasing power for life and unleashing the machineries of death, Cell, 2003, vol. 112, no. 4, pp. 481–490.
Ovakimov, V.N. and Yarmonenko, S.P., Adaptation to hypoxia as a factor modifying its radioprotective effect, Med. Radiol., 1974, vol. 19, no. 6, pp. 49–53.
Pistollato, F., Abbadi, S., Rampazzo, E., Viola, G., Della, Puppa A., Cavallini, L., Frasson, C., Persano, L., Panchision, D.M., and Basso, G., Hypoxia and succinate antagonize 2-deoxyglucose effect glioblastoma, Biochem. Pharmacol., 2010, vol. 80, no. 10, pp. 1517–1527.
Richardt, G., Lumpp, U., Haass, M., and Schömig, A., Propranolol inhibits nonexocytotic noradrenaline release in myocardial ischemia, Naunyn Schmiedebergs Arch. Pharmacol., 1990, vol. 341, nos. 1–2, pp. 50–55.
Schömig, A., Haass, M., and Richardt, G., Catecholamine release and arrhythmias in acute myocardial ischaemia, Eur. Heart J., 1991, vol. 12, suppl. F, pp. 38–47.
Schömig, A., Richardt, G., and Kurz, T., Sympatho-adrenergic activation of the ischemic myocardium and its arrhythmogenic impact, Herz, 1995, vol. 20, no. 3, pp. 169–186.
Sivaramakrishnan, S. and Ramasarma, T., Noradrenaline stimulates succinate dehydrogenase through beta-adrenergic receptors, Indian J. Biochem. Biophys., 1983a, vol. 1983, no. 20, pp. 1–16.
Sivaramakrishnan, S., Panini, S.R., and Ramasarma, T., Activation of succinate dehydrogenase in isolated mitochondria by noradrenaline, Indian J. Biochem. Biophys., 1983b, vol. 1983, no. 20, pp. 1–23.
Skrede, S., Effects of cystamine and cysteamine on the adenosine- triphosphatase activity and oxidative phosphorylation of rat-liver mitochondria, Biochem. J., 1966, vol. 98, no. 3, pp. 702–710.
Souvannakitti, D., Kumar, G.K., Fox, A., and Prabhakar, N.R., Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells, J. Neurophysiol., 2009, vol. 101, no. 6, pp. 2837–2846.
Ushakov, I.B., Abramov, M.M., Khunandov, L.L., and Zuev, V.G., Radioprotektory i gipoksiya: mekhanizmy kombinirovannoi zashchity (Radioprotectors and Hypoxia: Mechanisms of Combined Protection), Moscow: Vooruzhenie. Politika. Konversiya, 1996.
Vasin, M.V., Comparative characteristics of the modification of radiosensitivity of mice and rats by a hypoxic mixture, Radiobiologiya, 1986, vol. 26, no. 4, pp. 563–565.
Vasin, M.V., Classification of radioprotective agents as a reflection of the current state and prospects of development of radiation pharmacology?, Radiats. Biol. Radioekol., 2013, vol. 53, no. 5, pp. 459–467.
Vasin, M.V. and Ushakov, I.B., Comparative efficacy and the window of radioprotection for adrenergic and serotoninergic agents and aminothiols in experiments with small and large animals, J. Radiat. Res., 2015, vol. 56, no. 1, pp. 1–10.
Vasin, M.V., Antipov, V.V., Suvorov, N.N., Abramov, M.M., and Gorelova, N.V., Characteristics of the role of the hydroxyl group in serotonin in the pharmacological and antiradiation effect of serotonin, Radiobiologiya, 1984, vol. 24, no. 3, pp. 411–414.
Vasin, M.V., Suvorov, N.N., Abramov, M.M., and Gordeev, E.N., Changes in the therapeutic spectrum with respect to the pharmacological and radioprotective activity after O-alkylation of serotonin and 5(2-hydroxyethoxytryptamine), Radiobiologiya, 1987, vol. 27, no. 5, pp. 700–703.
Vasin, M.V., Petrova, T.V., and Koroleva, L.V., The effect of adrenaline on the cyclic nucleotides and succinate dehydrogenase activity, Fiziol. Zh. SSSR im. I.M. Sechenova, 1991, vol. 77, no. 4, pp. 106–108.
Vasin, M.V., Chernov, G.A., Koroleva, L.V., L’vova, T.S., Abramov, M.M., Antipov, V.V., and Suvorov, N.N., Mechanism of the radiation-protective effect of indralin, Radiats. Biol. Radioekol., 1996, vol. 36, no. 1, pp. 36–46.
Vasin, M.V., Antipov, V.V., Chernov, G.A., Abramov, M.M., Gavrilyuk, D.N., L’vova, T.S., and Suvorov, N.N., The role of the vasoconstrictor effect in realizing the radioprotective properties of indralin in experiments on dogs, Radiats. Biol. Radioekol., 1997, vol. 37, no. 1, pp. 46–55.
Vasin, M.V., Ushakov, I.B., Koroleva, L.V., and Antipov, V.V., The role of cell hypoxia in the effect of radiation protectors, Radiats. Biol. Radioekol., 1999, vol. 39, nos. 2–3, pp. 238–248.
Vasin, M.V., Ushakov, I.B., Semenova, L.A., and Kovtun, V.Yu., Pharmacologic analysis of the radiation-protecting effect of indraline, Radiats. Biol. Radioekol., 2001, vol. 41, no. 3, pp. 307–309.
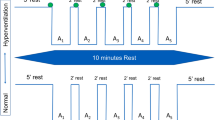
Vasin, M.V., Ushakov, I.B., Koroleva, L.V., and Stepanov, V.K., Participation of succinate dehydrogenase cell system in adaptive processes during human breathing with a hypoxic gas mixture, Aviakosm. Ekol. Med., 2002a, vol. 2002, no. 36, pp. 6–35.
Vasin, M.V., Ushakov, I.B., Koroleva, L.V., Lairov, I.A., and Radchenko, S.N., In vitro response of mitochondrial succinate oxidase system to epinephrine in human blood lymphocytes from health individuals and patients with neurocirculatory dystonia, Byull. Eksp. Biol. Med., 2002b, vol. 2002, no. 134, pp. 4–393.
Vasin, M.V., Ushakov, I.B., and Antipov, V.V., Potential role of catecholamine response to acute hypoxia in the modification of the effects of radioprotectors, Byull. Eksp. Biol. Med., 2015, vol. 159, no. 5, pp. 549–552.
Vladimirov, V.G., The effect of cystamine on oxidative phosphorylation in the spleen of irradiated rats, Byull. Eksp. Biol. Med., 1962, vol. 54, no. 11, pp. 55–58.
Wittenberger, T., Schaller, H.C., and Hellebrand, S., An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors, J. Mol. Biol., 2001, vol. 307, pp. 799–813.
Yang, L., Yu, D., Fan, H.H., Feng, Y., Hu, L., Zhang, W.Y., Zhou, K., and Mo, X.M., Triggering the succinate receptor GPR91 enhances pressure overload-induced right ventricular hypertrophy, Int. J. Clin. Exp. Pathol., 2014, vol. 7, no. 9, pp. 5415–5428.
Yarmonenko, S.P. and Epshtein, I.M., Oxygen effect and the intracellular oxygen content (the adaptation hypothesis), Radiobiologiya, 1977, vol. 17, no. 3, pp. 323–335.
Yarmonenko, S.P., Rampan, Yu.I., Karochkin, B.B., Berezhnova, L.I., Ovakimov, V.G., and Aupanetyan, G.M., Oxygen tension kinetics in critical organs under the effects of mexamine in comparison with its radiation-protective effect, Radiobiologiya, 1970, vol. 10, no. 6, pp. 700–705.
Yarmonenko, S.P., Vainson, A.A., and Magdon, E., Kislorodnyi effekt i luchevaya terapiya opukholei (Oxygen Effect and Radiation Therapy of Tumors), Moscow: Meditsina, 1980.
Yuhas, J.M., Proctor, J.O., and Smith, L.H., Some pharmacologic effects of WR-2721: their role in toxicity and radioprotection, Radiat. Res., 1973, vol. 54, pp. 222–233.
Zakharchenko, M.V., Zakharchenko, A.V., Khunderyakova, N.V., Tutukina, M.N., Simonova, M.A., Vasilieva, A.A., Romanova, O.I., Fedotcheva, N.I., Litvinova, E.G., Maevsky, E.I., Zinchenko, V.P., Berezhnov, A.V., Morgunov, I.G., Gulayev, A.A., and Kondrashova, M.N., Burst of succinate dehydrogenase and α-ketoglutarate dehydrogenase activity in concert with the expression of genes coding for respiratory chain proteins underlies shortterm beneficial physiological stress in mitochondria, Int. J. Biochem. Cell Biol., 2013, vol. 45, no. 1, pp. 190–200.
Zepeda, A.B., Pessoa, A., Castillo, R.L., Figueroa, C.A., Pulgar, V.M., and Farías, J.G., Cellular and molecular mechanisms in the hypoxic tissue: role of HIF-1 and ROS, Cell Biochem. Funct., 2013, vol. 31, no. 6, pp. 451–459.
Zherebchenko, P.G. and Suvorov, N.N., The relationship between the radioprotective and vasoconstrictor effects of indolylalkylamines, Radiobiologiya, 1963, vol. 3, pp. 595–602.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.V. Vasin, I.B. Ushakov, I.V. Bukhtiyarov, 2018, published in Izvestiya Akademii Nauk, Seriya Biologicheskaya, 2018, No. 1, pp. 83–92.
Rights and permissions
About this article
Cite this article
Vasin, M.V., Ushakov, I.B. & Bukhtiyarov, I.V. Stress Reaction and Biochemical Shock as Interrelated and Unavoidable Components in the Formation of High Radioresistance of the Body in Acute Hypoxia. Biol Bull Russ Acad Sci 45, 73–81 (2018). https://doi.org/10.1134/S1062359017060115
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359017060115