Abstract
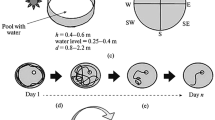
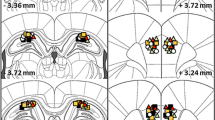
The involvement of the caudal hippocampus in spatial learning is presently uncertain, compared to the well established role of the dorsal region. Therefore voles (Clethrionomys glareolus) with large (about 1/3 of the whole hippocampus) caudal cytotoxic lesions were tested in the Morris water maze. A version of the test intended to measure long term spatial memory was used. The lesion inhibited the learning process, as well as reducing the accuracy of platform location memory at early stages of training. The data obtained indicate the involvement of this area in control of spatial learning in rodents.
Similar content being viewed by others
References
Amaral, D.G. and Witter, M.P., The Three-Dimensional Organization of the Hippocampal Formation: A Review of Anatomical Data, Neuroscience, 1989, vol. 31, pp. 571–591.
Bannerman, D.M., Yee, B.K., Good, M.A., et al., Double Dissociation of Function within the Hippocampus: A Comparison of Dorsal, Ventral, and Complete Hippocampal Cytotoxic Lesions, Behav. Neurosci., 1999, vol. 113, no. 6, pp. 1170–1188.
Bannerman, D.M., Rawlins, J.N., McHugh, S.B., et al., Regional Dissociations within the Hippocampus-Memory and Anxiety, Neurosci. Biobehav. Rev., 2004, vol. 28, no. 3, pp. 273–283.
Bast, T., Toward an Integrative Perspective on Hippocampal Function: From the Rapid Encoding of Experience to Adaptive Behavior, Rev. Neurosci., 2007, vol. 18, nos. 3–4, pp. 253–281.
Bast, T., Wilson, I.A., Witter, M.P., and Morris, R.G., From Rapid Place Learning to Behavioral Performance: A Key Role for the Intermediate Hippocampus, PLoS Biol., 2009, vol. 7, no. 4, p. e1000089.
Czerniawski, J., Yoon, T., and Otto, T., Dissociating Space and Trace in Dorsal and Ventral Hippocampus, Hippocampus, 2009, vol. 19, no. 1, pp. 20–32.
Deacon, R.M., Croucher, A., and Rawlins, J.N., Hippocampal Cytotoxic Lesion Effects on Species-Typical Behaviours in Mice, Behav. Brain Res., 2002, vol. 132, no. 2, pp. 203–213.
Fanselow, M.S. and Dong, H.W., Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures?, Neuron, 2010, vol. 65, no. 1, pp. 7–19.
Ferbinteanu, J., Ray, C., and McDonald, R.J., Both Dorsal and Ventral Hippocampus Contribute to Spatial Learning in Long-Evans Rats, Neurosci. Lett., 2003, vol. 345, no. 2, pp. 131–135.
Ferino, F., Thierry, A.M., and Glowinski, J., Anatomical and Electrophysiological Evidence for a Direct Projection from Ammon’s Horn to the Medial Prefrontal Cortex in the Rat, Exp. Brain Res., 1987, vol. 65, no. 2, pp. 421–426.
Hok, V., Lenck-Santini, P.P., Save, E., et al., A Test of the Time Estimation Hypothesis of Place Cell Goal-Related Activity, J. Integr. Neurosci., 2007, vol. 6, no. 3, pp. 367–378.
de Hoz, L., Knox, J., and Morris, R.G., Longitudinal Axis of the Hippocampus: Both Septal and Temporal Poles of the Hippocampus Support Water Maze Spatial Learning Depending on the Training Protocol, Hippocampus, 2003, vol. 13, no. 5, pp. 587–603.
Jung, M.W., Wiener, S.I., and McNaughton, B.L., Comparison of Spatial Firing Characteristics of Units in Dorsal and Ventral Hippocampus of the Rat, J. Neurosci., 1994, vol. 14, no. 12, pp. 7347–7356.
Kerr, K.M., Agster, K.L., Furtak, S.C., and Burwell, R.D., Functional Neuroanatomy of the Parahippocampal Region: The Lateral and Medial Entorhinal Areas, Hippocampus, 2007, vol. 17, no. 9, pp. 697–708.
Kjelstrup, K.G., Tuvnes, F.A., Steffenach, H.A., et al., Reduced Fear Expression after Lesions of the Ventral Hippocampus, Proc. Natl. Acad. Sci. USA, 2002, vol. 99, no. 16, pp. 10825–10830.
Kjelstrup, K.B., Solstad, T., Brun, V.H., et al., Finite Scale of Spatial Representation in the Hippocampus, Science, 2008, vol. 321, no. 5885, pp. 140–143.
Kuptsov, P.A., Pleskacheva, M.G., Voronkov, D.N., et al., Features of the c-Fos Gene Expression along the Hippocampal Rostro-Caudal Axis in Common Voles after Rapid Spatial Learning, Zh. Vyssh. Nervn. Deyat., 2005, vol. 55, no. 2, pp. 231–240.
Kuptsov, P.A., Pleskacheva, M.G., and Anokhin, K.V., Inhomogeneous Hippocampal Activation along the Rostrocaudal Axis in Mice after Exploration of Novel Environment, Zh. Vyssh. Nervn. Deyat., 2012, vol. 62, no. 1, pp. 43–55.
Loureiro, M., Lecourtier, L., Engeln, M., et al., The Ventral Hippocampus Is Necessary for Expressing a Spatial Memory, Brain Struct. Funct., 2012, vol. 217, no. 1, pp. 93–106.
McHugh, S.B., Fillenz, M., Lowry, J.P., et al., Brain Tissue Oxygen Amperometry in Behaving Rats Demonstrates Functional Dissociation of Dorsal and Ventral Hippocampus during Spatial Processing and Anxiety, Eur. J. Neurosci., 2011, vol. 33, no. 2, pp. 322–337.
Morris, R.G., Garrud, P., Rawlins, J.N., and O’Keefe, J., Place Navigation Impaired in Rats with Hippocampal Lesions, Nature, 1982, vol. 297, pp. 681–683.
Morris, R., Developments of a Water-Maze Procedure for Studying Spatial Learning in the Rat, J. Neurosci. Methods, 1984, vol. 11, no. 1, pp. 47–60.
Moser, E.I., Moser, M.B., and Andersen, P., Spatial Learning Impairment Parallels the Magnitude of Dorsal Hippocampal Lesions, but Is Hardly Present Following Ventral Lesions, J. Neurosci., 1993, vol. 13, no. 9, pp. 3916–3925.
Moser, M.B., Moser, E.I., Forrest, E., et al., Spatial Learning with a Minislab in the Dorsal Hippocampus, Proc. Natl. Acad. Sci. USA, 1995, vol. 92, no. 21, pp. 9697–9701.
Moser, M.B. and Moser, E.I., Functional Differentiation in the Hippocampus, Hippocampus, 1998, vol. 8, no. 6, pp. 608–619.
Moser, E.I., Kropff, E., and Moser, M.B., Place Cells, Grid Cells, and the Brain’s Spatial Representation System, Annu. Rev. Neurosci., 2008, vol. 31, pp. 69–89.
O’Keefe, J. and Nadel, L., The Hippocampus as a Cognitive Map, Oxford: Oxford Univ. Press, 1978.
Pleskacheva, M.G., Wolfer, D.P., Kupriyanova, I.F., et al., Hippocampal Mossy Fibers and Swimming Navigation Learning in Two Vole Species Occupying Different Habitats, Hippocampus, 2000, vol. 10, no. 1, pp. 17–30.
Poucet, B., Thinus-Blanc, C., and Muller, R.U., Place Cells in the Ventral Hippocampus of Rats, Neuroreport, 1994, vol. 5, no. 16, pp. 2045–2048.
Richmond, M.A., Yee, B.K., Pouzet, B., et al., Dissociating Context and Space within the Hippocampus: Effects of Complete, Dorsal, and Ventral Excitotoxic Hippocampal Lesions on Conditioned Freezing and Spatial Learning, Behav. Neurosci., 1999, vol. 113, no. 6, pp. 1189–1203.
Royer, S., Sirota, A., Patel, J., and Buzsaki, G., Distinct Representations and Theta Dynamics in Dorsal and Ventral Hippocampus, J. Neurosci., 2010, vol. 30, no. 5, pp. 1777–1787.
Silachev, D.N., Shram, S.I., Shakova, F.M., et al., Formation of Spatial Memory in Rats with Ischemic Lesions to the Prefrontal Cortex; Effects of a Synthetic Analog of ACTH(4-7), Neurosci. Behav. Physiol., 2009, vol. 39, no. 8, pp. 749–756.
Vandebroek, I., Bouche, K., D’Herde, K., et al., A Stereotaxic Atlas of the Forebrain of the Bank Vole (Clethrionomys glareolus), Brain Res. Bull., 1999, vol. 48, no. 6, pp. 555–567.
Vann, S.D., Brown, M.W., Erichsen, J.T., and Aggleton, J.P., Fos Imaging Reveals Differential Patterns of Hippocampal and Parahippocampal Subfield Activation in Rats in Response to Different Spatial Memory Tests, J. Neurosci., 2000, vol. 20, no. 7, pp. 2711–2718.
Vinogadova, O.S., Gippokamp i pamyat’ (Hippocampus and Memory), Moscow: Nauka, 1975.
Wolfer, D.P., Madani, R., Valenti, P., and Lipp, H.P., Extended Analysis of Path Data from Mutant Mice Using the Public Domain Software Wintrack, Physiol. Behav., 2001, vol. 73, no. 5, pp. 745–753.
Yartsev, M.M., Distinct or Gradually Changing Spatial and Nonspatial Representations along the Dorsoventral Axis of the Hippocampus, J. Neurosci., 2010, vol. 30, no. 23, pp. 7758–7760.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.V. Lebedev, D.V. Bezryadnov, R.M.J. Deacon, P.A. Kuptsov, V.M. Malygin, M.G. Pleskacheva, 2013, published in Izvestiya Akademii Nauk, Seriya Biologicheskaya, 2013, No. 2, pp. 197–205.
Rights and permissions
About this article
Cite this article
Lebedev, I.V., Bezryadnov, D.V., Deacon, R.M.J. et al. The effect of a caudal hippocampus lesion on learning in a Morris water maze in Bank Voles (Clethrionomys glareolus). Biol Bull Russ Acad Sci 40, 179–186 (2013). https://doi.org/10.1134/S1062359013020088
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359013020088