Abstract
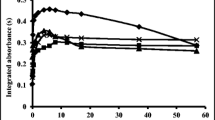
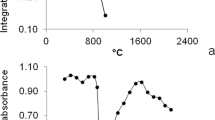
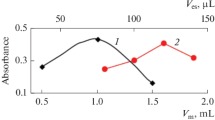
In this research, a preconcentration procedure was developed for the determination of silver in environmental samples using high-resolution continuum source graphite furnace atomic absorption spectrometry (HR-CS GFAAS). During the preconcentration step, bentonite was used as a cheap solid sorbent in ultrasound-assisted dispersive micro solid-phase extraction. The experimental parameters including pH of the sample solution, bentonite amount, and ultrasonication time as well as the main parameters of HR-CS GFAAS were investigated. The limit of detection was 0.01 µg/L, and the achieved preconcentration factor was 34. The relative standard deviation was 5%. The accuracy of this method was validated by analyses of NIST SRM 2709 (San Joaquin soil), NIST SRM 2711 (Montana soil), and NIST SRM 1643e (trace elements in water) certified reference materials. The proposed method was successfully applied for the determination of silver in soil and water samples.



Similar content being viewed by others
REFERENCES
The Silver Institute. http://www.silverinstitute.org. Accessed October 11, 2021.
Abou El-Nour, K.M.M., Eftaiha, A., Al-Warthan, A., and Ammar, R.A.A., Arab. J. Chem., 2010, vol. 3, p. 135.
Kabata-Pendias, A. and Mukherjee, A.B., Trace Elements from Soil to Human, Heidelberg: Springer, 2007.
Nordberg, G.F., Fowler, B.A., Nordberg, M., and Friberg, L., Handbook of the Toxicology of Metals, San Diego: Elsevier, 2007, 3rd ed.
Vandecasteele, C. and Block, C.B., Modern Methods for Trace Element Determination, Chichester: Wiley, 1993.
Jiang, X., Huang, K., Deng, D., Xia, H., Hou, X., and Zheng, Ch., TrAC, Trends Anal. Chem., 2012, vol. 39, p. 38.
Atkovska, K., Bliznakovska, B., and Ruseska, G., J. Chem. Technol. Metall., 2016, vol. 51, p. 215.
Mohellebi, F. and Lakel, F., Desalin. Water Treat., 2016, vol. 57, p. 6051.
Melichova, Z. and Hromada, L., Pol. J. Environ. Stud., 2013, vol. 22, p. 457.
Hong, S., Wen, Ch., He, J., Gan, F., and Ho, Y.S., J. Hazard. Mater., 2009, vol. 167, p. 630.
Wang, S., Dong, Y., He, M., Chen, L., and Yu, X., Appl. Clay Sci., 2009, vol. 43, p. 164.
Krawczyk, M., Akbari, S., Jeszka-Skowron, M., Pajootan, E., and Fard, F.S., J. Anal. At. Spectrom., 2016, vol. 31, p. 1505.
Krawczyk-Coda, M., Spectrochim. Acta, Part B, 2017, vol. 129, p. 21.
Ghiasvand, A.R., Moradi, F., Sharghi, H., and Hasaninejad, A.R., Anal. Sci., 2005, vol. 21, p. 387.
Hartmann, G., Hutterera, C., and Schuster, M., J. Anal. At. Spectrom., 2013, vol. 28, p. 567.
Fathabad, S.D. and Hasanvandi, S., Orient. J. Chem., 2012, vol. 28, p. 1311.
Ghiasvand, A., Shadabi, S., Hajipour, S., and Nasirian, A., Anal. Bioanal. Chem. Res., 2015, vol. 2, p. 60.
Taher, M.A., Daliri, Z., and Fazelirad, H., Chin. Chem. Lett., 2014, vol. 25, p. 649.
Ekinci, C. and Köklü, Ü., Spectrochim. Acta, Part B, 2000, vol. 55, p. 1491.
Ghanei-Motlagh, M., Fayazi, M., Taher, M.A., and Jalalinejad, A., Chem. Eng. J., 2016, vol. 290, p. 53.
Avila, A.K. and Curtius, A.J., J. Anal. At. Spectrom., 1994, vol. 9, p. 543.
Medved, J., Matúš, P., Bujdoš, M., and Kubová, J., Chem. Pap., 2006, vol. 60, p. 27.
Dadfarnia, S., Haji Shabani, A.M., and Gohari, M., Talanta, 2004, vol. 64, p. 682.
Kurfürst, U., Solid Sample Analysis: Direct and Slurry Sampling Using GF-AAS and ETV-ICP, Heidelberg: Springer, 1998.
Khan, S.A., Rehman, R., and Khan, M.A., Waste Manage., 1995, vol. 15, p. 271.
Currie, L.A., Pure Appl. Chem., 1995, vol. 67, p. 1699.
Rydberg, J., Solvent Extraction Principles and Practice, Revised and Expanded, Boca Raton: CRC Press, 2004, 2nd ed.
Funding
This work was supported the Ministry of Education and Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Krawczyk-Coda, M. Determination of Silver in Environmental Samples by High-resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry after Preconcentration on Bentonite. J Anal Chem 77, 1155–1161 (2022). https://doi.org/10.1134/S1061934822090076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934822090076