Abstract
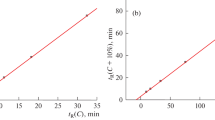
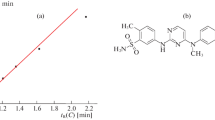
The formation of hydrates of inorganic compounds is well known and is widely discussed in the contemporary literature. The reasons for the insufficiently detailed characterization of numerous well-known hydrates of organic compounds are analyzed, the main of which is their instability. The CAS numbers assigned to many of these hydrates confirm interest in these hydrates. We found that the reversible formation of the hydrates of various organic compounds can be a reason for anomalous dependences of their retention parameters (tR) on the concentration of an organic eluent modifier (c) in reversed-phase high-performance liquid chromatography. The recurrent approximation of the retention parameters tR(c + Δc) = atR(c) + b (*), where Δc = const is a constant step of c, is the most effective way to detect these anomalies. Deviations of dependences like (*) from linearity are observed with compounds in which variations in c and, consequently, in the water content of the eluent (1 – c) affect the equilibrium of the reaction \({\text{X}} + n{{{\text{H}}}_{{\text{2}}}}{\text{O }} \rightleftarrows {\text{X}} \cdot n{{{\text{H}}}_{{\text{2}}}}{\text{O}}.\)


Similar content being viewed by others
REFERENCES
Griesser, U.J., in Polymorphism in the Pharmaceutical Industry, Hilfiket, R., Ed., New York: Wiley, 2006, ch. 8. https://doi.org/10.1002/3527607889.ch8
Spravochnik khimika (Chemist’s Handbook), vol. 2: Osnovnye svoistva neorganicheskikh i organicheskikh soedinenii (Basic Properties of Inorganic and Organic Compounds), Leningrad: Khimiya, 1971.
The Merck Index: An Encyclopedia of Chemicals and Drugs, Merck, 1976, 9th ed.
Svoistva organicheskikh soedinenii. Spravochnik (Properties of Organic Compounds: Handbook), Potekhin, A.A., Ed., Leningrad: Khimiya, 1984.
Handbook of Chemistry and Physics, Lide, D.R., Ed., Boca Raton: CRC, 2006.
Sangster, J., Octanol–Water Partition Coefficients: Fundamentals and Physical Chemistry, New York: Wiley, 1997.
Zenkevich, I.G., Olisov, D.A., Shafigullin, R.V., and Bulanova, A.V., Analitika Kontrol’, 2019, vol. 23, no. 3, p. 386. https://doi.org/10.15826/analitika.2019.23.3.013
Rawn, J.D. and Quellette, R., Organic Chemistry, New York: Academic, 2019, 2nd ed.
Zenkevich, I.G. and Nikitina, D.A., Abstracts of Papers, IV Vseross. konf. molodykh uchenykh “Mediko-biologicheskie aspekty khimicheskoi bezopasnosti” (IV All-Russian Conf. of Young Scientists on Medical and Biological Aspects of Chemical Safety), St. Petersburg, 2020.
Zenkevich, I.G. and Nikitina, D.A., Russ. J. Phys. Chem. A, 2021, vol. 95, no. 2, p. 395. https://doi.org/10.1134/S003602442102028X
Martin, R., Aust. J. Chem., 1954, vol. 7, no. 4, p. 400.
Zavitas, A.A., Coffiner, M., Wiseman, T., and Zavitas, L.R., J. Phys. Chem., 1970, vol. 74, no. 14, p. 2746.
Winkelman, L.G.M., Voorwinde, O.K., Ottens, M., Beenackers, A.A.C.M., and Janssen, L.P.B.M., Chem. Eng. Sci., 2002, vol. 57, p. 4067.
Bell, R.P. and Higginsen, W.C.E., Proc. R. Soc. London, Ser. A, 1949, vol. 197, no. 1047, p. 141.
Scheithauer, A., Grützner, T., Rijksen, C., Zollinger, D., and Thiel, W.R., AIChE J., 2015, vol. 61, no. 1, p. 177.
Wilson, G.J. and Davidson, D.W., Can. J. Chem., 1963, vol. 41, no. 2, p. 264.
Yamamuro, O., Kuratomi, N., Matsuo, T., and Suga, H., Solid State Commun., 1990, vol. 73, no. 4, p. 317.
McLain, S.E., Soper, A.K., and Luzar, A., J. Chem. Phys., 2007, vol. 127, 174515. https://doi.org/10.1063/1.2784555
Du, J., Kiang, D.-O., Li, D.-L., and Li, X.-J., J. Chem. Eng. Data, 2010, vol. 55, no. 10, p. 4532.
Edwards, H.G., Lawson, E., Matas, M., Shields, L., and York, P., J. Chem. Soc., Perkin Trans., 1997, no. 10, p. 1985.
Hédoux, A., Paccou, L., Derollez, P., and Guinet, Y., Int. J. Pharm., 2015, nos. 1–2, p. 331.
Suzuki, E., Shimomura, K., and Sekiguchi, K., Chem. Pharm. Bull., 1989, vol. 37, no. 1, p. 493.
Gould, P.L., Howard, J.R., and Oldenshaw, G.A., Int. J. Pharm., 1989, vol. 51, no. 3, p. 195.
Agbada, C.O. and York, P., J. Pharm. Pharmacol., 1990, vol. 42, no. S1, p. 76.
Rodríguez-Hornedo, N. and Wu, H.-J., Pharm. Res., 1991, vol. 8, p. 643.
Sun, C.C., Zhou, D., Grant, D.J.W., and Young, V.G., Acta Crystallogr., 2002, vol. 58, no. 4.
González-González, J.S., Zuñiga-Lemus, O., and Hernández-Galindo, M., IOSR J. Pharm., 2017, vol. 7, no. 5, p. 28.
Shcherbakov, A.A., Danilov, I.P., Sazhin, V.A., and Petrov, V.I., Theor. Exp. Chem., 1993, vol. 28, no. 4, p. 277.
Scott, H., J. Pharm. Sci., 1969, vol. 58, no. 8, p. 946.
Tester, J.W. and Wiegandt, H.F., AIChE J., 1969, vol. 15, no. 2, p. 239.
Nakayama, H. and Tahara, M., Bull. Chem. Soc. Jpn., 1973, vol. 46, p. 2965.
Gough, S.R., Ripmeester, J.A., and Davidson, D.W., Can. J. Chem., vol. 53, no. 15, p. 2216.
Kamran-Pirzaman, A., Pahlavanzadeh, H., and Mohammadi, A.H., J. Chem. Thermodyn., 2013, vol. 45, no. 21, p. 151.
Pchelkin, V.N. and Toryanik, A.I., J. Struct. Chem., 1991, vol. 32, no. 2, p. 227.
Braun, D., Tocher, D.A., Price, S.L., and Griesser, U.J., J. Phys. Chem., 2012, vol. 116, no. 3, p. 3361.
Buschmann, H.-J., Füldner, H.-H., and Knocke, W., Berichte, 1980, vol. 84, no. 1, p. 41.
ebi.ac.uk/chebi/searched.do?chebiId=63733. Accessed October 2020.
Heady, A.M., Worku, Z.A., Kumar, D., and Madi, A.M., Adv. Drug. Delivery Rev., 2017, vol. 117, p. 25.
Funabiki, K., Matsunaga, K., Nojiri, M., and Hashimoto, W., Org. Chem., 2003, vol. 68, no. 7, p. 2853.
Zenkevich, I.G., J. Phys. Chem. A, 2008, vol. 82, no. 6, p. 886.
Zenkevich, I.G., J. Chemom., 2009, vol. 23, p. 179.
Zenkevich, I.G., in Chemometrics in Chromatography, Komsta, L., Heyden, Y.V., and Sherma, J., Eds., London: CRC, 2018, p. 449.
Kobayashi, M. and Nishioka, K., J. Phys. Chem., 1987, vol. 91, no. 5, p. 1247.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Zenkevich, I.G., Nikitina, D.A. & Deruish, A. Formation and Chromatographic Detection of Organic Compound Hydrates. J Anal Chem 76, 493–502 (2021). https://doi.org/10.1134/S1061934821040146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934821040146