Abstract
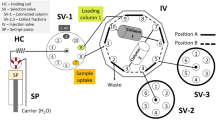
A method has been developed for the separation of lanthanides, Ce, Nd, Gd, La, Pr, Sm, Dy, Eu, Er, Ho, Yb, Tm, Tb and Lu, using oxalate form of Dowex-1, a reactive ion-exchanger. Lanthanides present in such samples as monazite sand, coal fly ash and sediment were separated from matrix at pH 2.5 in a column containing oxalate form of Dowex-1 resin. The samples have been decomposed in H2SO4 and taken in 0.36 M H2SO4 prior to separation. The lanthanides in the column were eluted with 2 M HNO3. Lanthanides present in the eluent were determined by inductively coupled plasma optical emission spectrometry (ICP−OES) and the recovery of analytes ranged from 92 to 105%. Matrix free solutions were analyzed for lanthanides by ICP−OES. The relative standard deviation was in the range of 4–7% and limits of detection were between 0.015–0.16 mg/kg. The developed procedure was applied to the separation and determination of lanthanides in a standard reference material NIST Coal Fly Ash 1633b, a lake sediment and monazite sand. The results obtained by the present method are in close agreement with certified values in case of certified reference material and microwave digestion method for other samples. Accuracy of other samples is ensured based on standard addition recoveries.


Similar content being viewed by others
REFERENCES
Bahramifar, N. and Yamini, Y., Anal. Chim. Acta, 2005, vol. 540, p. 325.
Cyber Riden. http://cyberraiden.wordpress.com/ 2012/04/22/rare-earth-elements-and-their-uses. Accessed February 2, 2018.
Rao, T. and Kala, R., Talanta, 2004, vol. 63, p. 949.
Shan, X.Q., Lian, J., and Wen, B., Chemosphere, 2002, vol. 47, p. 701.
Hubicki, Z. and Olszak, M., J. Chromatogr. A, 2002, vol. 955, p. 257.
Franus, W., Wiatros-Motyka, M.M., and Wdowin, M., Environ. Sci. Pollut. Res. Int., 2015, vol. 22, p. 9464.
Peterson, R., Heinrichs, M., Glier, J., Lane, A., and Taha, R., Proc. 2017 World of Coal Ash (WOCA) Conf., Lexington, 2017.
Peiravi, M., Ackah, L., Rajesh, G., Mohanty, M., Liu, J., Xu, B., Zhu, X., and Chen, L., Miner. Metall. Process., 2017, vol. 34, p. 170.
Pagano, G., Guida, M., Tommasi, F., and Oral, R., Ecotoxicol. Environ. Saf., 2015, vol. 115, p. 40.
Li, X., Chen, Z., Chen, Z., and Zhang, Y., Chemosphere, 2013, vol. 93, p. 1240.
Betlin, F.R.S. and Pozebon, D., J. Braz. Chem. Soc., 2010, vol. 21, p. 627.
Fisher, A. and Kara, D., Anal. Chim. Acta, 2016, vol. 935, p. 1.
Rao, T. and Kara, D., Talanta, 2004, vol. 63, p. 949.
Zawisza, B., Pytlakowska, K., Feist, B., Polowniak, M., Kita, A., and Sitko, R., J. Anal. At. Spectrosc., 2011, vol. 26, p. 2373.
Xie, F., An Zhang, T., Dreisinger, D., and Doyle, F., Miner. Eng., 2014, vol. 56, p. 10.
dos Santos Depoi, F., Bentlin, F.R.S., Flores Ferrao, M., and Pozebon, D., Anal. Methods, 2012, vol. 4, p. 2809.
Zereen, F., Yilmaz, V., and Arslan, Z., Microchem. J., 2013, vol. 110, p. 178.
Karadas, C. and Kara, D., Water, Air, Soil Pollut., 2014, vol. 225, p. 2192.
Karadas, C., Kara, D., and Fisher, A., Anal. Chim. Acta, 2011, vol. 689, p. 184.
Sungur, S. and Akseli, A., J. Chromatogr. A, 2000, vol. 874, p. 311.
Venkateswarlu, G., Sahayam, A.C., Chaurasia, S.C., and Mukherjee, T., Talanta, 2006, vol. 68, p. 748.
ACKNOWLEDGMENTS
The authors wish to acknowledge constant support and encouragement from Dr. Sunil Jai Kumar, Head, NCCCM, BARC, Hyderabad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gumma Venkateswarlu, Mamatha, P.R., Thangavel, S. et al. Determination of Lanthanides in Coal Fly Ash, Sediment and Monazite Sand by Inductively Coupled Plasma Optical Emission Spectrometry After Separation Using Oxalate form of Ion-Exchange Resin . J Anal Chem 76, 180–184 (2021). https://doi.org/10.1134/S1061934821020155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934821020155