Abstract
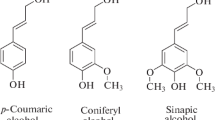
The study compares the efficiency of laser desorption/ionization (LDI) of six dimeric compounds, corresponding to the most important types of bonds between monomeric structural fragments of lignin macromolecules, on three types of different stainless steel target plates. It was found that ground steel target plates demonstrate the best results because of the uniform crystallization of analytes and good surface coverage. The most important ionization mechanisms for lignin oligomers with conjugated structures and increased acidity, along with protonation (cationization), involve the formation of molecular ions due to direct photoionization. For all of the studied compounds, partial in-source decay was observed, the contribution of which became significant at high laser fluence. The LDI method was successfully used to obtain mass spectra of low-molecular-weight products of the depolymerization of technical lignins. It was shown that the use of hybrid mass spectrometers with a quadrupole ion trap leads to an increase in the percentage of signals of protonated molecules in the mass spectra of lignin depolymerization products because of the occurrence of side ion-molecular reactions in the plume and ion trap.






Similar content being viewed by others
REFERENCES
Vanholme, R., Demedts, B., Morreel, K., et al., Plant Physiol., 2010, vol. 153, no. 3, p. 895.
Karak, N., in Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials, Pacheco-Torgal, F., Ivanov, V., Karak, N., et al., Eds., Cambridge: Woodhead, 2016, p. 333.
Abhilash, M. and Thomas, D., in Biopolymer Composites in Electronics, Sadasivuni, K.K., Cabibihan, J.J., Ponnamma, D., et al., Eds., Amsterdam: Elsevier, 2016, p. 405.
Boerjan, W., Ralph, J., and Baucher, M., Annu. Rev. Plant Biol., 2003, vol. 54, p. 519.
Heitner, C., Dimmel, D., and Schmidt, J., Lignin and Lignans: Advances in Chemistry, Boca Raton, FL: CRC, 2010.
Albishi, T., Mikhael, A., Shahidi, F., et al., Rapid Commun. Mass. Spectrom., 2019, vol. 33, no. 6, p. 539.
Chen, C.L., in Methods in Lignin Chemistry, Lin, S.Y., and Dence, C.W., Eds., Berlin: Springer; 1992, p. 301.
Lundquist, K., Appl. Polym. Symp., 1976, vol. 28, p. 1393.
Bayerbach, R. and Meier, D., J. Anal. Appl. Pyrolysis, 2009, vol. 85, nos. 1–2, p. 98.
Dimmel, D.R. and Boon, J.J., J. Wood Chem. Technol., 2001, vol. 21, no. 1, p. 19.
Del Rio, J.C., Rencoret, J., Prinsen, P., et al., J. Agric. Food Chem., 2012, vol. 60, no. 23, p. 5922.
Del Rio, J.C., Gutierrez, A., Romero, J., et al., J. Anal. Appl. Pyrolysis, 2001, vols. 58–59, p. 425.
Wen, J.L., Sun, S.L., Xue, B.L., et al., Materials, 2013, vol. 6, no. 1, p. 359.
Morreel, K., Kim, H., Lu, F., et al., Anal. Chem., 2010, vol. 82, no. 19, p. 8095.
Kosyakov, D.S., Ul’yanovskii, N.V., Anikeenko, E.A., et al., Rapid Commun. Mass Spectrom., 2016, vol. 30, no. 19, p. 2099.
Banoub, J., Delmas, G.H., Joly, N., et al., J. Mass Spectrom., 2015, vol. 50, no. 1, p. 5.
Nielen, M.W.F., Mass Spectrom. Rev., 1999, vol. 18, no. 5, p. 309.
Yoshioka, K., Ando, D., and Watanabe, T., Phytochem. Anal., 2011, vol. 23, no. 3, p. 248.
Kosyakov, D.S., Ul’yanovskii, N.V., Sorokina, E.A., et al., J. Anal. Chem., 2014, vol. 69, no. 14, p. 1344.
Kosyakov, D.S., Anikeenko, E.A., Ul’yanovskii, N.V., et al., Anal. Bioanal. Chem., 2018, vol. 410, no. 28, p. 7429.
Bayerbach, R., Nguyen, V.D., Schurr, U., et al., J. Anal. Appl. Pyrolysis, 2006, vol. 77, no. 2, p. 95.
Kosyakov, D.S., Ipatova, E.V., Krutov, S.M., et al., J. Anal. Chem., 2017, vol. 72, no. 14, p. 1396.
Ivakhnov, A.D., Shavrina, I.S., Kosyakov, D.S., et al., Russ. J. Appl. Chem., 2020, vol. 93, no. 1, p. 99.
Kosyakov, D.S., Sorokina, E.A., Ul’yanovskii, N.V., et al., J. Anal. Chem., 2016, vol. 71, no. 13, p. 1221.
Srzić, D., Martinović, S., Paša Tolic, L.J., et al., Rapid Commun. Mass Spectrom., 1995, vol. 9, no. 3, p. 245.
Zakis, G.F., Sintez model’nykh soedinenii lignina: Metodiki (Synthesis of Model Compounds of Lignin: Procedures), Riga: Zinatne, 1980, p. 182.
Kolářová, L., Prokeš, L., Kučera, L., et al., J. Am. Soc. Mass Spectrom., 2017, vol. 28, no. 3, p. 419.
Sládková, K., Houška, J., and Havel, J., Rapid Commun. Mass Spectrom., 2009, vol. 23, no. 19, p. 3114.
López-Nicolás, J.M. and García-Carmona, F., J. Agric. Food Chem., 2008, vol. 56, no. 17, p. 7600.
Ragnar, M., Lindgren, C.T., and Nilvebrant, N.-O., J. Wood Chem. Technol., 2000, vol. 20, no. 3, p. 277.
Kosyakov, D.S., Khoroshev, O.Yu., Anikeenko, E.A., et al., J. Anal. Chem., 2019, vol. 74, no. 14, p. 1390.
ACKNOWLEDGMENTS
This work was performed using instrumentation of the “Arktika” Core Facility Center of the Northern (Arctic) Federal University.
Funding
This work was supported by the Russian Science Foundation, project no. 18-73-10138.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated by E. Rykova
ADDITIONAL INFORMATION
E.A. Anikeenko.: ORCID ID 0000000211711305
N.V. Ul’yanovskii: ORCID ID 0000000347969313
D.S. Kosyakov: ORCID ID 0000000152236857
I.S. Shavrina: ORCID ID 0000000332723185
Rights and permissions
About this article
Cite this article
Anikeenko, E.A., Ul’yanovskii, N.V., Shavrina, I.S. et al. Laser Desorption/Ionization of Low-Molecular-Weight Lignin Oligomers. J Anal Chem 75, 1814–1824 (2020). https://doi.org/10.1134/S1061934820140038
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934820140038