Abstract
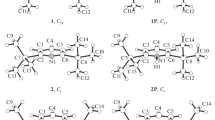
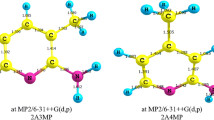
Accurate calculations of the structure and properties of analytes and their ions are of great interest to the theory and practice of mass spectrometry, ion mobility spectrometry, and related methods. In this work, using accurate quantum chemical methods, we computed the structure of neutral and protonated pyridine, 2-methyl-, 4-methyl-, 2,4-dimethyl-, 2,6-dimethyl-, and 2,4,6-trimethylpyridine molecules and also of proton-bound dimers of pyridine and 2,4-dimethylpyridine. Two stable conformers of the 2,4-dimethylpyridine proton-bound dimer are found. An accurate and economical method is proposed for calculating proton affinity and gas-phase basicity with the calculation error close to the experimental one. The values of proton affinity and gas-phase basicity for 2,4,6-trimethylpyridine are computed. Reduced mobilities of protonated molecules and proton-bound dimers are calculated by the trajectory method in different versions. A calculation version is proposed that allows mobility computation with the error close to the experimental one and adequately reproduces small differences in the mobility of isomers. The versatility of the chosen B3PW91−D3BJ/def2−TZVP method of the density functional theory with the inclusion of dispersion correction is shown. The method (in combination with B2GP−PLYP−D3BJ/def2−TZVPPD calculations) ensures accurate calculations of the molecular structure, proton affinity, and gas-phase basicity, and also charges on atoms for computing ion mobility.


Similar content being viewed by others
REFERENCES
Lebedev, A.T., Mass-spektrometria v organicheskoi khimii (Mass Spectrometry in Organic Chemistry), Moscow: Tekhnosfera, 2015.
Mata, F., Quintana, M.J., and Sørensen, G.O., J. Mol. Struct., 1977, vol. 42, p. 1.
Wörmke, S., Brendel, K., Andersen, U., et al., Mol. Phys., 2004, vol. 102, nos. 14–15, p. 1625.
Srinivasan, R., Feenstra, J.S., Park, S.T., et al., Science, 2005, vol. 307, no. 5709, p. 558.
Császár, A.G., Demaison, J., and Rudolph, H.D., J. Phys. Chem. A, 2015, vol. 119, no. 9, p. 1731.
Sunner, J., Nicol, G., and Kebarle, P., Anal. Chem., 1988, vol. 60, no. 13, p. 1300.
Eiceman, G.A., and Karpas, Z., Ion Mobility Spectrometry, Boca Raton, FL: CRC, 2005.
Nishikaze, T., and Takayama, M., Rapid Commun. Mass Spectrom., 2006, vol. 20, no. 3, p. 376.
Grechnikov, A.A., Borodkov, A.S., Alimpiev, S.S., et al., J. Anal. Chem., 2013, vol. 68, no. 1, p. 19.
Hunter, E.P.L., and Lias, S.G., J. Phys. Chem. Ref. Data, 1998, vol. 27, no. 3, p. 413.
Gurinov, A.A., Denisov, G.S., Borissova, A.O., et al., J. Phys. Chem. A, 2017, vol. 121, no. 45, p. 8697.
Karpas, Z., Anal. Chem., 1989, vol. 61, no. 7, p. 684.
Karpas, Z., Berant, Z., and Shahal, O., J. Am. Chem. Soc., 1989, vol. 111, no. 16, p. 6015.
Ghira, G.-B., Raţiu, I.-A., and Bocoş-Binţinţan, V., Environ. Eng. Manage. J., 2013, vol. 12, no. 2, p. 251.
Fernández-Maestre, R., Int. J. Mass Spectrom. Ion Processes, 2017, vol. 421, p. 8.
Laakia, J., Adamov, A., Jussila, M., et al., J. Am. Soc. Mass Spectrom., 2010, vol. 21, no. 9, p. 1565.
Ewing, R.G., Eiceman, G.A., Harden, C.S., et al., Int. J. Mass Spectrom. Ion Processes, 2006, vols. 255–256, p. 76.
Thomas, C.L.P., Rezgui, N.D., Kanu, A.B., et al., Int. J. Ion Mobility Spectrom., 2002, vol. 5, no. 1, p. 31–36.
Kanu, A.B., Hill, H.H., Jr., Gribb, M.M., et al., J. Environ. Monit., 2007, vol. 9, p. 51.
Kaur-Atwal, G., O’Connor, G., Aksenov, A.A., et al., Int. J. Ion Mobility Spectrom., 2009, vol. 12, no. 1, p. 1.
Granovsky, A.A., Firefly, version 8.0.1. http://classic. chem.msu.su/gran/firefly/index.html. Accessed July 15, 2014.
Grimme, S., Ehrlich, S., and Goerigk, L., J. Comput. Chem., 2011, vol. 32, no. 7, p. 1456.
Karton, A., Tarnopolsky, A., Lamère, J.-F., et al., J. Phys. Chem. A, 2008, vol. 112, no. 50, p. 12868.
Catalán, J., Mó, O., Pérez, P., et al., J. Am. Chem. Soc., 1979, vol. 101, no. 22, p. 6520.
Hillebrand, C., Klessinger, M., Eckert-Maksic, M., et al., J. Phys. Chem., 1996, vol. 100, no. 23, p. 9698.
Nguyen, V.Q., and Turecek, F., J. Mass Spectrom., 1997, vol. 32, no. 1, p. 55.
Golec, B., Das, P., Bahou, M., et al., J. Phys. Chem. A, 2013, vol. 117, no. 50, p. 13680.
Boys, S.F. and Bernardi, F., Mol. Phys., 1970, vol. 19, no. 4, p. 553.
Xantheas, S.S., J. Chem. Phys., 1996, vol. 104, no. 21, p. 8821.
Lewars, E.G., Computational Chemistry, Dordrecht: Springer, 2011, 2nd ed.
Larriba, C. and Hogan, C.J., J. Phys. Chem. A, 2013, vol. 117, no. 19, p. 3887.
Larriba, C. and Hogan, C.J., J. Comp. Phys., 2013, vol. 251, p. 344.
Ouyang, H., Larriba-Andaluz, C., Oberreit, D., et al., J. Am. Soc. Mass Spectrom., 2013, vol. 24, no. 12, p. 1833.
Mesleh, M.F., Hunter, J.M., Shvartsburg, A.A., et al., J. Phys. Chem., 1996, vol. 100, no. 40, p. 16082.
Takaya, K., Kaneko, T., Tanuma, H., et al., Int. J. Ion Mobility Spectrom., 2016, vol. 19, no. 4, p. 227.
Lebedev, A.V., J. Anal. Chem., 2019, vol. 74, no. 13, p. 1325.
Wu, T., Derrick, J., Nahin, M., et al., J. Chem. Phys., 2018, vol. 148, no. 7, 074102.
Bader, R.F.W. and Matta, C.F., J. Phys. Chem. A, 2004, vol. 108, no. 40, p. 8385.
Keith, T.A., AIMAll, version 15.09.27, 2015.
Kanu, A.B. and Hill, H.H., Jr., Talanta, 2007, vol. 73, no. 4, p. 692.
Karpas, Z. and Berant, Z., J. Phys. Chem., 1989, vol. 93, no. 8, p. 3021.
Stone, J.A., Int. J. Ion Mobility Spectrom., 2002, vol. 5, no. 2, p. 19.
Mäkinen, M., Sillanpää, M., Viitanen, A.-K., et al., Talanta, 2011, vol. 84, no. 1, p. 116.
Campuzano, I., Bush, M.F., Robinson, C.V., et al., Anal. Chem., 2012, vol. 84, no. 2, p. 1026.
Pápai, I. and Jáncsó, G., J. Phys. Chem. A, 2000, vol. 104, no. 10, p. 2132.
Roithová, J. and Exner, O., J. Phys. Org. Chem., 2001, vol. 14, no. 11, p. 752.
Ruusuvuori, K., Kurten, T., Ortega, I.K., et al., Atmos. Chem. Phys., 2013, vol. 13, no. 20, p. 10397.
Lapouge, C. and Cavagnat, D., J. Phys. Chem. A, 1998, vol. 102, no. 43, p. 8393.
Chen, P.C. and Chang, F.M., Int. J. Quantum Chem., 2000, vol. 77, no. 4, p. 772.
East, A.L.L., Liu, H., Lima, E.C., et al., J. Chem. Phys., 2000, vol. 112, no. 1, p. 167.
Borst, D.R. and Pratt, D.W., J. Chem. Phys., 2000, vol. 113, no. 9, p. 3658.
Gardner, A.M., Green, A.M., Tame-Reyes, V.M., et al., J. Chem. Phys., 2013, vol. 138, no. 13, 134303.
Del Bene, J.E., J. Am. Chem. Soc., 1977, vol. 99, no. 11, p. 3617.
Ajito, K., Takahashi, M., and Ito, M., Chem. Phys. Lett., 1989, vol. 158, nos. 3–4, p. 193.
Makowski, M., Sadowski, R., Augustin-Nowacka, D., et al., J. Phys. Chem. A, 2001, vol. 105, no. 27, p. 6743.
Blanco, F., O’Donovan, D.H., Alkorta, I., et al., Struct. Chem., 2008, vol. 19, no. 2, p. 339.
Bouchoux, G., Mass Spectrom. Rev., 2007, vol. 26, no. 25, p. 775.
Ishida, H., Z. Naturforsch., A: Phys. Sci., 2000, vol. 55, nos. 9–10, p. 769.
Zeroka, D. and Jensen, J.O., J. Mol. Struct.: THEOCHEM, 1998, vol. 425, no. 3, p. 181.
Zeroka, D., Jensen, J.O., and Samuels, A.C., J. Mol. Struct.: THEOCHEM, 1999, vol. 465, nos. 2–3, p. 119.
Holroyd, L.F. and van Mourik, T., Chem. Phys. Lett., 2007, vol. 442, nos. 1–3, p. 42–46.
Chan, B., Del Bene, J.E., and Radom, L., Mol. Phys., 2009, vol. 107, nos. 8–12, p. 1095.
Melikova, S.M., Rutkowski, K.S., Gurinov, A.A., et al., J. Mol. Struct., 2012, vol. 1018, p. 39.
Attah, I.K., Platt, S.P., Meot-Ner (Mautner), M., et al., J. Chem. Phys., 2014, vol. 140, no. 11, p. 114313-1.
Ebrahimi, A., Habibi-Khorasani, S.M., and Jahantab, M., Comput. Theor. Chem., 2011, vol. 966, nos. 1–3, p. 31.
Pham-Tran, N.N., Bouchoux, G., Delaere, D., et al., J. Phys. Chem. A, 2005, vol. 109, no. 12, p. 2957.
Hauck, B.C., Harden, C.S., and McHugh, V.M., Int. J. Ion Mobility Spectrom., 2018, vol. 21, no. 4, p. 105.
Meot-Ner (Mautner), M., Chem. Rev., 2005, vol. 105, no. 1, p. 213.
Reed, A.E., Weinhold, F., Curtiss, L.A., et al., J. Chem. Phys., 1986, vol. 84, no. 10, p. 5687.
Funding
This work had no sponsor support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflict of interest.
Additional information
Translated by E. Rykova
Rights and permissions
About this article
Cite this article
Lebedev, A.V. Pyridine and Methylpyridines: Calculations of the Structure, Proton Affinity, Gas-Phase Basicity, and Mobility of Protonated Molecules and Proton-Bound Dimers. J Anal Chem 75, 1719–1730 (2020). https://doi.org/10.1134/S1061934820130079
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934820130079