Abstract
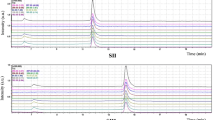
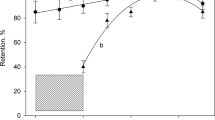
The concentration of mercury in suspensions of sediments is determined by electrothermal atomic absorption spectrometry (ET-AAS) using a novel chemical modifier (ChM) based on activated carbon treated by iron(II) formate. The efficiency of chemical modifiers based on activated carbon, also modified by Ag(I), Au(III), Pd(II), Co(II), and Ni(II) compounds is studied. The optimum conditions are found for the temperature and time program of the atomizer; they prevent mercury losses at the stage of drying sample suspensions and also exclude spectral interferences at the stage of atomization and measurement of the analytical signals. The minimum characteristic mass of mercury (52 pg) is reached using an iron-containing chemical modifier based on activated carbon. The conditions of ET-AAS analysis using this ChM are tested on certified reference materials of sediments and a sample selected near the sea port of Temryuk (Sea of Azov). The limit of detection found by the 3s-test was 0.05 mg/kg and the relative standard deviation was 5% at the concentration of mercury in the sample 149 mg/kg.





Similar content being viewed by others
REFERENCES
Gworek, B., Bemowska-Kałabun, O., Kijeńska, M., and Wrzosek-Jakubowska, J., Water, Air, Soil Pollut., 2016, vol. 227, p. 371.
Horowitz, A.J., A Primer on Sediment-Trace Element Chemistry, Boca Raton, FL: CRC, 1991.
Amde, M., Yin, Y., Zhang, D., and Liu, J., Chem. Speciation Bioavailability, 2016, vol. 28, p. 51.
Naik, R.M., Agarwal, A., and Prasad, S., Spectrochim. Acta, Part A, 2009, vol. 74, p. 887.
Bansal, N., Vaughan, J., Boullemant, A., and Leong, T., The determination of trace mercury in environmental samples: A review, in Chemeca 2013: Challenging Tomorrow, Barton: ACT, 2013, p. 771.
Wu, P., He, L., Zheng, C., Hou, X., and Stur-geon, R.E., J. Anal. At. Spectrom., 2010, vol. 25, p. 1217.
Matusiewicz, H. and Sturgeon, R., Spectrochim. Acta, Part B, 1996, vol. 51, p. 377.
Biester, H. and Nehrke, G., Fresenius’ J. Anal. Chem., 1997, vol. 358, p. 446.
Almeida, I.L.S., Oliveira, M.D.R., Silva, J.B.B., and Coelho, N.M.M., Microchem. J., 2016, vol. 124, p. 326.
Matusiewicz, H. and Sturgeon, R.E., Appl. Spectrosc. Rev., 2012, vol. 47, p. 41.
Coufal, P. and Kom, J., J. Anal. Chem., 2014, vol. 69, p. 1123.
Clevenger, W.L., Smith, B.W., and Winefordner, J.D., Crit. Rev. Anal. Chem., 1997, vol. 27, p. 1.
Costa Ferreira, S.L., Miró, M., Galvão Paranhos da Silva, E., Domingues Matos, G., Sanches dos Reis, P., Cardoso Brandao, G., Lopes dos Santos, W.N., Tavares Duarte, A., Goreti Rodrigues Vale, M., and Oliveira Araujo, R.G., Appl. Spectrosc. Rev., 2010, vol. 45, p. 44.
Sardans, J., Montes, F., and Peñuelas, J., Soil Sediment Contam., 2011, vol. 20, p. 447.
Burylin, M.Yu. and Pupyshev, A.A., J. Anal. Chem., 2017, vol. 72, no. 9, p. 935.
Bulska, E., Kandler, W., and Hulanicki, A., Spectrochim. Acta, Part B, 1996, vol. 51, p. 1263.
Silva, A.F., Welz, B., and Curtius, A.J., Noble metals as permanent chemical modifiers for the determination of mercury in environmental reference materials, Spectrochim. Acta, vol. 57, nos. 9–10, p. 2031.
López-García, I., Sánchez-Merlos, M., and Hernaández-Córdoba, M., Spectrochim. Acta, Part B, 1997, vol. 52, p. 2085.
Burylin, M.Yu., Malykhin, S.E., and Galai, E.F., J. Anal. Chem., 2015, vol. 70, no. 4, p. 380.
Burylin, M.Yu., Malykhin, S.E., and Galai, E.F., Inorg. Mater., 2016, vol. 52, no. 14, p. 1383.
Burylin, M.Yu., Temerdashev, Z.A., and Burylin, S.Yu., J. Anal. Chem., 2006, vol. 61, no. 1, p. 37.
Burylin, M.Yu., Temerdashev, Z.A., Pupyshev, A.A., Kaunova, A.A., and Obogrelova, S.A., J. Appl. Spectrosc., 2006, vol. 73, no. 5, p. 760.
ISO 11466:1995. Soil Quality: Extraction of Trace Elements in Aqua Regia, 1995. https://www.iso.org/standard/19418.html. Accessed February 24, 2019.
Bermejo-Barrera, P., Moreda-Piñeiro, J., Moreda-Piñeiro, A., and Bermejo-Barrera, A., Anal. Chim. Acta, 1994, vol. 296, p. 181.
Cal-Prieto, M.J., Felipe-Sotelo, M., Carlosena, A., Andrade, J.M., López-Mahía, P., Muniategui, S., and Prada, D., Talanta, 2002, vol. 56, p. 1.
Karadjova, I., Mandjukov, P., Tsakovsky, S., Stratis, J.A., and Zachariadis, G.A., J. Anal. At. Spectrom., 1995, vol. 10, p. 1065.
Zelinková, H., Červenka, R., and Komárek, J., Stabilizing agents for calibration in the determination of mercury using solid sampling electrothermal atomic absorption spectrometry, Sci. World J., 2012, vol. 2012, 439 875. https://doi.org/10.1100/2012/439875
L’vov, B.V., Nikolaev, V.G., Norman, E.A., Polzik, L.K., and Mojica, M., Spectrochim. Acta, Part B, 1986, vol. 418, no. 1978, p. 1043.
ACKNOWLEDGMENTS
This work was performed using scientific equipment of “Ecological and Analytical Center” Core Facility Center of the Kuban State University, unique identifier RFMEFI59317X0008.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 19-03-00181_a) and by the Ministry of Education and Science of the Russian Federation (contract no. 4.2612.2017/PCh).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Rykova
Rights and permissions
About this article
Cite this article
Burylin, M.Y., Romanovskiy, K.A., Temerdashev, Z.A. et al. Determination of Mercury in Sediments by Slurry Sampling Electrothermal Atomic Absorption Spectrometry. J Anal Chem 74, 1184–1191 (2019). https://doi.org/10.1134/S1061934819120037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934819120037