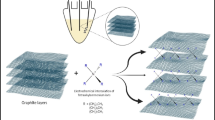
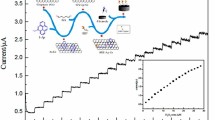
Abstract
A simple electrochemical method for detecting glutathione reductase was developed in this work. It was observed that the impedance of electrode increased obviously after the electrode modification by glutathione, and the value of impedance was closely related to the reduced glutathione (GSH) concentration. Based on the fact that glutathione reductase (GR) could catalyze oxidized glutathione (GSSG) to GSH rapidly in the presence of NADPH (β-nicotinamide adenine dinucleotide 2'-phosphate reduced), and as GSH was immobilized on the gold electrode surface, the impedance increased drastically. Meanwhile, it was found that the impedance was correlated with the activity of GR, and a chemical equation was obtained based on the relationship between the impedance and enzymatic activity. The range of enzymatic activity that could be measured at 0.005‒0.5 U by using this assay, and the detection limit was 0.005 U (1 U means reduction of 1.0 μmol GSSG per min at pH 7.2 at 25°C). The enzymatic activity of GR obtained by this method was compared with those obtained by colorimetric detection, and the results showed that the new method is reliable. Therefore, the new method is highly sensitive with convenience consuming time within 20 min to complete a test, thus showing a promising potential of being applied in medicine.







Similar content being viewed by others
REFERENCES
Wang, M., Sun, J., Xue, F., Shang, F., Wang, Z., and Tan, T., Appl. Biochem. Biotechnol., 2012, vol. 168, p. 198.
Valdovinos-Flores, C. and Gonsebatt, M.E., Neurochem. Int., 2012, vol. 61, p. 405.
Schettler, V., Wieland, E., Methe, H., Schuff-Werner, P., and Müller, G.A., Nephrol. Dial. Transplant., 1998, vol. 13, p. 2588.
Trivedi, M.S., Deth, R., and Zhang, Y., FASEB J., 2016, vol. 30, p. 289.
Ahmadpoor, P., Eftekhar, E., and Nourooz-Zadeh, J., Iran. J. Kidney Dis., 2009, vol. 3, p. 22.
Giustarini, D., Dalle-Donne, I., and Lorenzini, S., Free Radical Biol. Med., 2012, vol. 53, p. 907.
Couto, N., Wood, J., Barber, J., and Barber, J., Free Radical Biol. Med., 2016, vol. 95, p. 27.
Prast-Nielsen, S., Huang, H.H., and Williams, D.L., Biochim. Biophys. Acta, 2011, vol. 1810, p. 1262.
Gokturk, H., Ulusu, N.N., Gok, M., Tuncay, E., Can, B., and Turan, B., Mol. Cell. Biochem., 2014, vol. 395, p. 177.
Christophe, B., Holger, B., Heiner, R.S., and Davioud-Charvet, E., J. Med. Chem., 2004, vol. 47, p. 5972.
Ke, Z., Yu, Z., and Huang, Q., Plasma Process. Polym., 2013, vol. 10, p. 181.
Park, H.W. and Kim, J.D., J. Ind. Eng. Chem., 2009, vol. 15, p. 578.
Yin, G., Xin, X., Song, C., Chen, X., Zhang, J., Wu, S., Li, R., Liu, X., and Lu, X., Plant. Physiol. Biochem., 2014, vol. 80, p. 1.
Treger, R.S., Cook, A., Rai, G., Maloney, D.J., Simeonov, A., Jadhav, A., Thomas, C.J., Williams, D.L., Cappello, M., and Vermeire, J.J., Int. J. Parasitol., 2012, vol. 2, p. 171.
Maity, D. and Govindaraju, T., Org. Biomol. Chem., 2013, vol. 11, p. 2098.
Timur, S., Odaci, D., Dincer, A., Zihnioglu, F., and Telefoncu, A., Talanta, 2008, vol. 74, p. 1492.
Fernández, I., Araque, E., Martínez-Ruiz, P., di Pierro, P., Villalonga, R., and Pingarrón, J.M., Electrochem. Commun., 2014, vol. 40, p. 13.
Tietze, F., Anal. Biochem., 1969, vol. 27, p. 502.
Jiang, H., Su, X., and Zhang, Y., Anal. Chem., 2016, vol. 88, p. 4766.
Karacan, M.S., Tunç, T., and Oruç, H., Anal. Methods, 2015, vol. 7, p. 5142.
Rahman, I., Kode, A., and Biswas, S.K., Nat. Protoc., 2006, vol. 1, p. 3159.
Jiang, H., Su, X., Zhang, Y., Zhou, J., Fang, D., and Wang, X., Anal. Chem., 2016, vol. 88, p. 4766.
Wang, T., Su, W., Xiao, Z., Hao, S., Li, Y., and Hu, J., Analyst, 2015, vol. 140, p. 5176.
Massey, V. and Williams, C.H., J. Biol. Chem., 1965, vol. 240, p. 4470.
Ju, J., Zhang, R., and Chen, W., Sens. Actuators, B, 2016, vol. 228, p. 66.
Karacan, M.S., Tunç, T., and Oruç, H., Anal. Methods, 2015, vol. 7, p. 5142.
Lanfranchi, D.A., Belorgey, D., and Müller, T., Org. Biomol. Chem., 2012, vol. 10, p. 4795.
ACKNOWLEDGMENTS
This work was supported in part by the Research Foundation of Education Bureau of Hunan Province, China (16A227), Hunan Provincial Natural Science Foundation of China (2018JJ3869), Science and Technology Innovation Foundation of Graduate Students in Hunan Province (CX2016B3). National Natural Science Foundation of China (21 305 164).
Author information
Authors and Affiliations
Contributions
Ge Ning contributed to the data processing and language modification.
Corresponding authors
Rights and permissions
About this article
Cite this article
Yaohui Wu, Jiang, L., Ning, G. et al. A Sensitive and Simple Impedance Sensing Strategy for Glutathione and Glutathione Reductase Activity Detection. J Anal Chem 74, 505–512 (2019). https://doi.org/10.1134/S1061934819050101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934819050101