Abstract
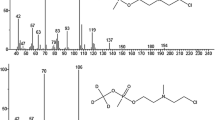
We propose an approach to the identification of alkyl radicals attached to the ester oxygen atom in a series of highly toxic organophosphorus compounds, based on the revealed regularities in the formation of mass spectra of monofunctional RX compounds (R is alkyl radical and X is functional group). The method includes the extraction of the hydrocarbon subspectrum from the mass spectrum of the analyte and its comparison with the hydrocarbon subspectra of the known monofunctional compounds. Alkyl radicals are identified using simple metrics that characterize the coincidence of hydrocarbon subspectra. The results of the identification of alkyl (cycloalkyl) radicals in a series of O-alkyl (cycloalkyl) alkylphosphonofluoridates, and O-alkyl (cycloalkyl) N,N-dialkylphosphoramidocyanidates show that ≥95% of the structures of the radicals of these compounds are within 10 positions of the search result list.


Similar content being viewed by others
REFERENCES
Tkachuk, Yu.V., Morozik, Yu.I., and Dudkin, A.V., Russ. J. Gen. Chem., 2015, vol. 85, no. 3, p. 556.
Morozik, Yu.I., Dudkin, A.V., Rybal’chenko, I.V., and Smirnov, K.N., Russ. J. Gen. Chem., 2016, vol. 86, no. 10, p. 2295.
Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction, GE 92-61926, Paris, 1993.
OPCW Central Analytical Database, e-OCAD ver.16, Technical Secretariat of the Organization for the Prohibition of Chemical Weapons, 2014.
Chemical Weapons Convention Chemicals Analysis: Sample Collection, Preparation, and Analytical Methods, Mesilaakso, M., Ed., Wiley, 2005, p. 9.
Lebedev, A.T., Morozik, Yu.I., Myasoedov, B.F., Rybal’chenko, I.V., and Fomenko, P.V., Mass-Spektrom., 2007, vol. 4, no. 4, p. 255.
Dudkin, A.V., Morozik, Yu.I., and Terent’ev, A.G., Certificate of State Registration of the Computer Program no. 2015613423, Russian Federation, Unit “Determination of the Molecular Formula of Monofunctional Compounds by Their Electron Ionization Mass Spectra” (MolForm 1.0), 2015.
NIST/EPA/NIH, NIST Mass Spectral Library, Natl. Inst. Standards Technol., US Secretary of Commerce, 2014.
Stein, S.E. and Scott, D.R., J. Am. Soc. Mass Spectrom., 1994, vol. 5, p. 859.
Kerber, A., Meringer, M., and Ruker, C., Croat. Chem. Acta, 2006, vol. 79, p. 449.
Morozik, Yu.I., Smirnov, A.O., and Galyaev, G.V., Russ. J. Gen. Chem., 2011, vol. 81, no. 10, p. 2088.
Morozik, Yu.I., Galyaev, G.V., and Smirnov, A.O., Russ. J. Gen. Chem., 2010, vol. 80, no. 8, p. 1593.
ACKNOWLEDGMENTS
The study was supported by the Russian Science Foundation, project no. 15-13-10005, granted to the Kostroma State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Dudkin, A.V., Morozik, Y.I., Rybal’chenko, I.V. et al. Determination of the Structure of Alkyl Radicals in a Series of Highly Toxic Organophosphorus Compounds Using Mass Spectrometry Databases and Library Search. J Anal Chem 73, 1275–1281 (2018). https://doi.org/10.1134/S1061934818130038
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934818130038