Abstract
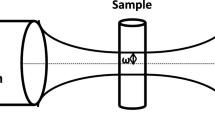
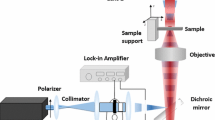
Thermal lens spectrometry in a coaxial configuration is used for the direct determination of adsorbates on a planar surface of polyethylene terephthalate (PET). A possibility of the direct measurement of the rate of adsorption from solutions and the determination of the parameters of the adsorbed layer is demonstrated by the example of an investigation of the adsorption of iron(II) tris(1,10-phenantrolinate) on a PET surface. The adsorption isotherm of iron(II) tris(1,10-phenantrolinate) on the PET surface is described by the Langmuir equation and is linear in the concentration range in solution from 0.02 to 0.7 mM. The method for calculating the thermal perturbation in surface-absorbing solids was used to interpret the results of the adsorption study, and a possibility of determining iron(II) tris(1,10-phenantrolinate) on the surface at a level smaller than a monolayer was shown. Thermal lens spectrometry enables the determination of the absorption of the surface layer at a level up to 5 × 10–5 absorbance units, which corresponds to the surface concentration of iron(II) tris(1,10-phenanthrolinate) 2 × 10–13 mol/cm2. Using the example of the adsorption of 4-(2-pyridylazo) resorcinol on the PET surface, it is demonstrated that, in the case of strong absorption of the surface layer, the thermal destruction of substance and the deformation of the substrate may occur. A local increase in temperature in the layer is also confirmed by theoretical calculations.
Similar content being viewed by others
References
Liu, M. and Franko, M., Crit. Rev. Anal. Chem., 2014, vol. 44, no. 4, p.328.
Bialkowski, S.E., Photothermal Spectroscopy Methods for Chemical Analysis, New York: Wiley, 1996.
Nedosekin, D.A., Saranchina, N.V., Sukhanov, A.V., Gavrilenko, N.A., Mikheev, I.V., and Proskurnin, M.A., Appl. Spectrosc., 2013, vol. 67, no. 7, p.709.
Korte, D. and Franko, M., Int. J. Thermophys., 2014, vol. 35, no. 12, p. 2352.
Malacarne, L.C., Astrath, N.G.C., Lukasievicz, G.V.B., Lenzi, E.K., Baesso, M.L., and Bialkowski, S.E., Appl. Spectrosc., 2011, vol. 65, no. 1, p.99.
Kononets, M.Yu, Cand. Sci. (Chem.) Dissertation, Moscow: Moscow State Univ., 2005.
Najmoddin, N. and Khosroshahi, M.E., Nucl. Instrum. Methods Phys. Res., Sect. A, 2015, vol. 774, p.1.
Capeloto, O.A., Lukasievicz, G.V.B., Zanuto, V.S., Herculano, L.S., Souza Filho, N.E., Novatski, A., Malacarne, L.C., Bialkowski, S.E., Baesso, M.L., and Astrath, N.G.C., Appl. Opt., 2014, vol. 53, no. 33, p. 7985.
Saranchina, N.V., Sukhanov, A.V., Nedosekin, D.A., Gavrilenko, N.A., and Proskurnin, M.A., J. Anal. Chem., 2011, vol. 66, no. 6, p.623.
Proskurnin, M.A. and Kononets, M.Yu., Russ. Chem. Rev., 2004, vol. 73, no. 12, p. 1143.
Wang, Z.-G., Wan, L.-S., and Xu, Z.-K., J. Membr. Sci., 2007, vol. 304, nos. 1–2, p.8.
Gavrilenko, N.A. and Saranchina, N.V., J. Anal. Chem., 2010, vol. 65, no. 2, p.148.
Gavrilenko, N.A. and Saranchina, N.V., J. Anal. Chem., 2009, vol. 64, no. 3, p.226.
Nedosekin, D.A., Proskurnin, M.A., and Kononets, M.Y., Appl. Opt., 2005, vol. 44, no. 29, p. 6296.
Schweitzer, M.A. and Power, J.F., Appl. Spectrosc., 1994, vol. 48, p. 1054.
Shen, J. and Snook, R.D., J. Appl. Phys., 1993, vol. 73, no. 10, p. 5286.
Proskurnin, M.A., Volkov, D.S., Gor’kova, T.A., Bendrysheva, S.N., Smirnova, A.P., and Nedosekin, D.A., J. Anal. Chem., 2015, vol. 70, no. 3, p.249.
Proskurnin, M.A., Photothermal spectroscopy, in Laser Spectroscopy for Sensing, Baudelet, M., Ed., Cambridge: Woodhead, 2014, p.313.
Proskurnin, M.A., Nedosekin, D.A., Volkov, D.S., Mikheev, I.V., and Filichkina, V.A., RF Patent 2615912, Byull. Izobret., 2017, no.11.
Kononets, M.Y., Proskurnin, M.A., Bendrysheva, S.N., and Chernysh, V.V., Talanta, 2001, vol. 53, no. 6, p. 1221.
Gladyshev, V.P., Levitskaya, S.A., and Fillipova, L.M., Analiticheskaya khimiya rtuti (Analytical Chemistry of Mercury), Moscow: Nauka, 1974.
Pons, M., Nonell, S., García-Moreno, I., Costela, Á., and Sastre, R., Appl. Phys. B: Lasers Opt., 2002, vol. 75, nos. 6–7, p.687.
Elperin, T. and Rudin, G., Heat Mass Transfer, 2010, vol. 46, no. 7, p. 717.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © D.A. Nedosekin, I.V. Mikheev, D.S. Volkov, M.A. Proskurnin, 2018, published in Zhurnal Analiticheskoi Khimii, 2018, Vol. 73, No. 7, pp. 498–507.
Rights and permissions
About this article
Cite this article
Nedosekin, D.A., Mikheev, I.V., Volkov, D.S. et al. Determination of Adsorbates on the Surface of Polymer with Low Absorption Capacity by Thermal Lens Spectrometry. J Anal Chem 73, 641–649 (2018). https://doi.org/10.1134/S1061934818070146
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934818070146