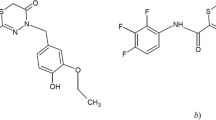
Abstract
A method for the determination of moxifloxacin in human blood plasma has been developed and validated by reversed-phase HPLC on a YMC-Triart C18 (150 × 2.0 mm, 3 μm) column with mass spectrometric detection. Sample preparation has been performed by solid-phase extraction on Waters Sep-Pak cartridges. The recovery of moxifloxacin was 90 ± 3%. A quadrupole mass analyzer with electrospray ionization has been used for detection at m/z 402.2 and 362.2 for moxifloxacin and ofloxacin (internal standard), respectively. The lower limit of the analytical range was 1 ng/mL. The developed method has been used for the pharmacokinetic study of moxifloxacin.
Similar content being viewed by others
References
Sousa, J., Alves, G., Campos, G., and Fortuna, A., J. Chromatogr. B: Biomed. Sci. Appl., 2013, vol. 930, p. 104.
Nguyen, H., Grellet, J., Ba, B., Quentin, C., and Saux, M., J. Chromatogr. B: Biomed. Sci. Appl., 2004, vol. 810, p. 77.
Schekina, E.G., Provisor, 2007, no. 21. www.provisor.com.ua/archive/2007/N21/shekina.php. Accessed January 16, 2014.
Stass, H. and Dalhoff, A., J. Chromatogr. B: Biomed. Sci. Appl., 1997, vol. 702, p. 163.
Trindade, M., Silva, G., and Ferreira, V., Microchem. J., 2005, vol. 81, p. 209.
Wagenlehner, F., Kees, F., Weidner, W., Wagenlehner, C., and Naber, K., Int. J. Antimicrob. Agents, 2008, vol. 31, p. 21.
Justinger, C., Schilling, M., Kees, M., Kauffels, A., Hirschmann, K., Kopp, B., Kees, F., and Kollmar, O., Int. J. Antimicrob. Agents, 2012, vol. 39, p. 505.
Moller, J., Stass, H., Heinig, R., and Blaschke, G., J. Chromatogr. B: Biomed. Sci. Appl., 1998, vol. 716, p. 325.
Kontou, P., Manika, K., Chatzika, K., Papaioannou, M., Sionidou, M., Pitsiou, G., and Kioumis, I., Int. J. Antimicrob. Agents, 2013, vol. 42, p. 262.
Kumar, A. and Ramachandran, G., J. Chromatogr. B: Biomed. Sci. Appl., 2009, vol. 877, p. 1205.
Laban-Djurdjevic, A., Jelikic-Stankov, M., and Djurdjevic, P., J. Chromatogr. B: Biomed. Sci. Appl., 2006, vol. 844, p. 104.
Lemoine, T., Breilh, D., Ducint, D., Dubrez, J., Jougon, J., Velly, J., and Saux, M., J. Chromatogr. B: Biomed. Sci. Appl., 2000, vol. 742, p. 247.
Liang, H., Kays, M., and Sowinski, K., J. Chromatogr. B: Biomed. Sci. Appl., 2002, vol. 772, p. 53.
Smet, J., Boussery, K., Colpaert, K., Sutter, P., Paepe, P., Decruyenaere, J., and Bocxlaer, J., J. Chromatogr. B: Biomed. Sci. Appl., 2009, vol. 877, p. 961.
Ulu, S., J. Pharm. Biomed. Anal., 2007, vol. 43, p. 320.
Vishwanathan, K., Bartlett, M., and Stewart, J., J. Pharm. Biomed. Anal., 2002, vol. 30, p. 961.
Xu, Y., Li, D., Liu, X., Li, Y., and Lu, J., J. Chromatogr. B: Biomed. Sci. Appl., 2010, vol. 878, p. 3437.
Zhang, L., Li, L., Shi, W., Liu, S., Liang, X., Ye, Z., Wang, W., Zhang, B., Li, R., Chen, Y., Yu, C., Zhuo, L., and Wang, X., Int. J. Antimicrob. Agents, 2013, vol. 42, p. 244.
Kumar, A., Sudha, V., Srinivasan, R., and Ramachandran, G., J. Chromatogr. B: Biomed. Sci. Appl., 2011, vol. 879, p. 3663.
Chan, K., Chu, K., Lai, W., Choy, K., Wang, C., Lam, D., and Pang, C., Anal. Biochem., 2006, vol. 353, p. 30.
Al-Ghannam, S., Spectrochim. Acta, Part A, 2008, vol. 69, p. 1188.
Dhillon, V. and Chaudhary, A., Pharm. Meth., 2010, vol. 2, p. 54.
Motwani, S., Khar, R., Ahmad, F., Chopra, S., Kohli, K., and Talegaonkar, S., Anal. Chim. Acta, 2007, vol. 582, p. 75.
Djurdjevic, P., Ciric, A., Djurdjevic, A., and Stankov, M., J. Pharm. Biomed. Anal., 2009, vol. 50, p. 117.
Ba, B., Etienne, R., Ducint, D., Quentin, C., and Saux, M., J. Chromatogr. B: Biomed. Sci. Appl., 2001, vol. 754, p. 107.
Szultka, M., Krzeminski, R., Jackowski, M., and Buszewski, B., J. Chromatogr. B: Biomed. Sci. Appl., 2013, vol. 940, p. 66.
Vu, D., Koster, R., Alffenaar, J., Brouwers, J., and Uges, D., J. Chromatogr. B: Biomed. Sci. Appl., 2011, vol. 879, p. 1063.
Guidance for Industry Bioanalytical Method Validation, U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM), 2001.
Guideline on Bioanalytical Method Validation, European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP), 2011.
Rukovodstvo po ekspertize lekarstvennykh sredstv (Guidance for Examination of Pharmaceutical Preparations), Moscow: Grif i K, 2013, vol. 1.
Yaroshenko, D.V., Grigor’ev, A.V., Sidorova, A.A., and Kartsova, L.A., J. Anal. Khim., 2013, vol. 68, no. 9, p. 801.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.S. Yaroshenko, A.Ya. Khaimenov, A.V. Grigor’ev, A.A. Sidorova, 2015, published in Zhurnal Analiticheskoi Khimii, 2015, Vol. 70, No. 7, pp. 745–753.
Rights and permissions
About this article
Cite this article
Yaroshenko, I.S., Khaimenov, A.Y., Grigor’ev, A.V. et al. A chromatographic-mass spectrometric method for the determination of moxifloxacin in blood plasma for pharmacokinetic studies. J Anal Chem 70, 860–868 (2015). https://doi.org/10.1134/S1061934815050160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934815050160