Abstract
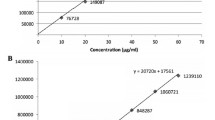
A simple, sensitive and rapid high performance liquid chromatographic method was developed and validated for the simultaneous determination of potassium clavulanate and cefadroxil in synthetically prepared tablets. Chromatographic separation and detection was carried out on a C-18 column using 0.05 M potassium dihydrogen phosphate buffer (pH 5.0) and acetonitrile in the ratio of 94: 06 (v/v) as mobile phase at wavelength of 225 nm. The method was linear in the concentration range of 3.75–22.5 μg/mL for potassium clavulanate and 15–90 μg/mL for cefadroxil. The flow rate was 1.0 mL/min and the total analysis time was less than 10 min. The mean recoveries was found to be greater than 99% with RSD less than 1.0%. The proposed method was validated by performing linearity, recovery, specificity, robustness, LOD/LOQ and within-day and between-day precision. The chromatographic results obtained from the synthetically prepared tablets show that the method is highly precise and accurate for the simultaneous quantitation of clavulanate potassium and cefadroxil.
Similar content being viewed by others
References
Elektronnyi resurs (Electronic Resource), www.rxlist.com/cgi/generic/cefixime.htm
Elektronnyi resurs (Electronic Resource), www.drugs.com
Elektronnyi resurs (Electronic Resource), http://cdsco.nic.in/index.html
Khan, I.U., Sharif, S., Ashfaq, M., and Asghar, M.N., J. AOAC Int., 2008, vol. 91, no. 4, p. 744.
The United States Pharmacoepial Convention 12601, USP 30-NF 25, Rockville, MD, 2007.
Makchit, J., Upalee, S., Thongpoon, C., Liawruangrath, B., and Liawrungrath, S., Anal. Sci., 2006, vol. 89, p. 591.
Samanidou, V.F., Hapeshi, E.A., and Papadoyannis, I.N., J. Chromatogr. B, 2003. vol.788. p.147.
El-Gindy, A., El Waily, A.F., and Bedair, M.F., J. Pharm. Biomed. Anal., 2000, vol. 23, p. 341.
Wu, Z., Guo, W.B., Zhang, Q.G., Ni, K.Y., and Lin, Y.S., Se Pu, 1999, vol. 17, p. 518.
Hsu, M.C., Chang, Y.W., and Lee, Y.T., J. Chromatogr., 1992, vol. 609, p. 181.
Gorski, R.J., Plasz, A.C., Elrod, L., Yoder, J., and White, L.B., Pharm. Res., 1991, vol. 8, p. 1525.
McAteer, J.A., Hiltke, M.F., Silber, B.M., and Faulkner, R.D., Clin. Chem., 1987, vol. 33, no. 10, p. 1788.
Lindgren, K., J. Chromatogr. B, 1987, vol. 413, p. 347.
Li, C., Geng, Q., Nicolau, D.P., and Nightingale, C.H., J. Chromatogr. B, 2003, vol. 794, no. 2, p. 227.
Hoizey, G., Lamiable, D., Frances, C., Trenque, T., Kaltenbach, M., Denis, J., and Millart, H., J. Pharm. Biomed. Anal., 2002, vol. 30, no. 3, p. 661.
Pajchel, G., Pawlowski, K., and Tyski, S., J. Pharm. Biomed. Anal., 2002, vol. 29, nos. 1–2, p. 75.
Tsou, T.L., Wu,.R., Young, C.D., and Wang, T.M., J. Pharm. Biomed. Anal., 1997, vol. 15, no. 8, p. 1197.
Jehl, F., Monteil, H., and Brogard, J.M., Pathol. Biol. (Paris), 1987, vol. 35, p. 702.
Foulstone M. and Reading, C., Antimicrobs Agents Chemother., 1982, vol. 22, p. 753.
Haginaka, J., Nakagawa, T., Nishino, Y., and Uno, T., J. Antibiot., (Tokyo), 1981, vol. 34, p. 1189.
Reyns, T., De Baere, S., Croubles, S., and De Backer, P., J. Mass Spectrom., 2006, vol. 41, p. 1414.
Yoon, K.H., Lee, S.Y., Kim, W., Park, J.S., and Kim, H.J., J. Chromatogr. B, 2004, vol. 813, nos. 1–2, p. 121.
Aghazadeh, A. and Kazemifard, G., J. Pharm. Biomed. Anal., 2001, vol. 25, no. 2, p. 325.
Shah, A.J., Adlard, M.W., and Stride, J.D., J. Pharm. Biomed. Anal., 1990, vol. 8, no. 5, p. 437.
Note for Guidance on Validation of Analytical Procedures: Methodology, Geneva: ICH, 2006.
Author information
Authors and Affiliations
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sharif, S., Khan, I.U., Ashfaq, M. et al. Development and validation of a high performance liquid chromatographic method for the simultaneous determination of potassium clavulanate and cefadroxil in synthetically prepared tablets. J Anal Chem 65, 1029–1034 (2010). https://doi.org/10.1134/S1061934810100072
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934810100072