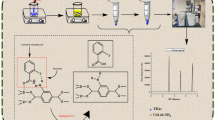
Abstract
Newly synthesized 2-propylpiperidine-1-carbodithioate (2-PPC) was used for the extraction of Cr(III), Ni(II), and Zn(II) from various water samples. In the present investigation, the use of a syringe loaded with sorbent for the separation and enrichment of Cr(III), Ni(II), and Zn(II) prior to their determination by inductively coupled plasma-atomic emission spectrometry (ICP-AES) was proposed to substitute the batch and column techniques. The described method was compared with the column technique with respect to fastness, simplicity, recovery, and risk of contamination. The syringe was loaded with 1.0 g of sorbent in order to retain the analyte elements. Next, 7.0 mL of sample solution (pH 5.0 ± 0.2) was drawn into the syringe in 15 s and discharged over 15 s. Then, an eluent (3.0 M HCl) was drawn into the syringe and ejected back to desorb the analyte elements. At the optimum conditions, the percentage recoveries of Cr(III), Ni(II), and Zn(II) were in the range of 94.50 to 99.62% with a standard deviation (S.D.) of 0.03%. The elements could be concentrated by drawing and discharging several portions of sample successively and eluting only one time. The detailed study of various interferences proved the method to be highly selective. The risk of contamination is less than that with the column technique. The method was successfully applied to the determination of Cr(III), Ni(II), and Zn(II) in spiked and natural water samples. The results obtained are in good agreement with those obtained by the reported methods at the 95% confidence level.
Similar content being viewed by others
References
Kozono, S., Kakashi, R., and Haraguchi, H., Anal. Sci., 2000, vol. 16, p. 69.
Keil, O., Dahmen, J., and Volmer, D.A., Fresenius’ J. Anal. Chem., 1999, vol. 364, p. 694.
Konar, B. and Basu, S., Fresenius’ J. Anal. Chem., 2002, vol. 348, p. 281.
Ozcan, M., Akman, S., and Ozeroglu, C., Anal. Lett., 2002, vol. 35, p. 1075.
Bakircioglu, Y., Seren, G., and Akman, S., Spectrochim. Acta, B., 2000, vol. 55, p. 1129.
Terada, K., Inove, A., Inamura, J., and Kiba, T., Bull. Chem. Soc. Jpn., 1977, vol. 5, p. 1060.
Soylak, M. and Dogan, M., Anal. Lett., 1996, vol. 29, p. 635.
Ralph, I.K., The Chemistry of Silica, New York: Wiley-Interscience, 1979, part 6, p. 622.
Cesur, H., Turk. J. Chem., 2003, vol. 27, p. 307.
Saracoglu, S., Soylak, M., and Elic, L., Acta. Chim. Slov., 2003, vol. 50, p. 807.
Ramesh, A., Ram Mohan, K., Seshaiah, K., and Jeya Kumar, N.D., Anal. Lett., 2001, vol. 34, no. 2, p. 219.
Alexandrova, A. and Arpadjan, S., Analyst, 1993, vol. 118, p. 309.
Balaji, T., Ph.D. Thesis, S.V. Univ., Tirupati, India, 1993.
Townshend, A. and Jackwerth, E., Pure. Appl. Chem., 1989, vol. 61, p. 1643.
Pai, S.-Ch., Whung, P.-Y., and Lai, R.-L., Anal. Chim. Acta, 1988, vol. 211, p. 257.
Avery, T.W., Chakrabarty, Ch., and Thompson, J.J., Appl. Spectrosc., 1990, vol. 44, p. 1690.
Manzoori, J.L. and Shemirani, F., J. Anal. At. Spectrosc., 1995, vol. 10, no. 10, p. 881.
Cerrato, O., Jimenez, De Blas, M.C., Perez, O., Pavon, J.L., and Cordero, M.B., J. Anal. At. Spectrom., 1998, vol. 13, p. 547.
Paleologos, E.K., Stalikas, C.D., Tzouwarakarayannis, S.M., Pilidis, G.A., and Karayannis, M.I., J. Anal. At. Spectrom., 2000, vol. 15, p. 287.
Giokas, D.L., Paleologas, E.K., Tzouwarakarayanni, S.M., and Karayannis, M.I., J. Anal. At. Spectrom., 2001, vol. 16, p. 521.
Soylak, M. and Elic, L., Int. J. Environ. Anal. Chem., 1997, vol. 66, p. 51.
Second Annual Report on Carcinogens, U.S. Environmental Protection Agency, NTP, 1981, p. 43.
Standard Methods for Examination of Water and Waste Water, 19th ed., Washington, DC: APHA, AWWA, and WEF, 1995.
Author information
Authors and Affiliations
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Suvardhan, K., Suresh Kumar, K., Rekha, D. et al. Novel solid-phase extraction and preconcentration technique coupled with ICP-AES for the determination of Cr(III), Ni(II), and Zn(II) in various water samples. J Anal Chem 62, 336–341 (2007). https://doi.org/10.1134/S1061934807040077
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1134/S1061934807040077