Abstract
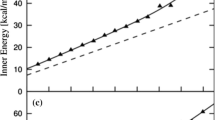
The stability of spherical nanoclusters of methane and carbon dioxide hydrates in the environment of supercooled water has been studied by the molecular dynamics method under the isochoric and isobaric conditions. The process of system melting has been considered within a temperature range of 180–280 K and at pressures of 1, 50, and 100 atm (under isobaric conditions). It has been shown that clusters of CO2 hydrate melt at temperatures lower than clusters of CH4 hydrate do. The difference between the melting temperatures of the hydrates is about 40 K, which is explained by the higher solubility of carbon dioxide in water. The diffusion coefficients calculated for water and the gases attest to different mechanisms of melting their hydrates. The stability of the hydrates under the isochoric conditions appears to be lower than that under the isobaric conditions. For simulation under isobaric conditions, changes in the pressure and the degree of carbon dioxide filling have no effect on the position of the range of melting of hydrate nanoclusters.







Similar content being viewed by others
REFERENCES
Boswell, R., Schoderbek, D., Collett, T.S., Ohtsuki, S., White, M., and Anderson, B.J., Energ. Fuels, 2017, vol. 31, p. 140.
Uddin, M. and Coombe, D., J. Phys. Chem. C, 2014, vol. 118, p. 1921.
Striolo, A., Mol. Phys., 2019. https://doi.org./10.1080/00268976.2019.1646436
English, N.J. and Macelroy, J.M.D., Chem. Eng. Sci., 2015, vol. 121, Art no. 133.
Costandy, J., Michalis, V.K., Tsimpanogiannis, I.N., Stubos, A.K., and Economou, I.G., J. Chem. Phys., 2015, vol. 143, Art no. 094506.
Smirnov, G.S. and Stegailov, V.V., J. Chem. Phys., 2012, vol. 136, Art no. 044523.
Miguez, J.M., Conde, M.M., Torre, J.-P., Blas, F.J., Pineiro, M.M., and Vega, C., J. Chem. Phys., 2015, vol. 142, p. 124505.
Liu, Y., Zhao, J., and Xu, J., Comput. Theor. Chem., 2012, vol. 991, p. 165.
Uddin, M. and Coombe, D., J. Phys. Chem. C, 2014, vol. 118, p. 1971.
Bagherzadeh, S.A., Alavi, S., Ripmeester, J., and En-glezos, P., J. Chem. Phys., 2015, vol. 142, Art no. 214701.
Sarupria, S. and Debenedetti, P.G., J. Phys. Chem. A, 2011, vol. 115, p. 6102.
Baez, L.A. and Clancy, P., Ann. N. Y. Acad. Sci., 1994, vol. 715, p. 177.
English, N.J., Johnson, J.K., and Taylor, C.E., J. Chem. Phys., 2005, vol. 123, Art no. 244503.
Subbotin, O.S., Belosludov, V.R., Brodskaya, E.N., Piotrovskaya, E.M., and Sizov, V.V., Russ. J. Phys. Chem., 2008, vol. 82, p. 1303.
Brodskaya, E.N. and Sizov, V.V., Colloid J., 2009, vol. 71, p. 589.
Brodskaya, E.N. and Sizov, V.V., Colloid J., 2013, vol. 75, p. 366.
Abraham, M.J., Murtola, T., Schulz, R., Pall, S., Smith, J.C., Hess, B., and Lindahl, E., SoftwareX, 2015, vol. 1, p. 19.
Van der Spoel, D., Lindahl, E., Hess, B., Groenhof, G., Mark, A.E., and Berendsen, H.J.C., J. Comput. Chem., 2008, vol. 26, p. 1701.
Jorgensen, W.L., Madura, J.D., and Swenson, C.J., J. Am. Chem. Soc., 1984, vol. 106, p. 6638.
Potoff, J.J. and Siepmann, J.I., AIChE J., 2001, vol. 47, p. 1676.
Abascal, J.L.F., Sanz, E., García Fernández, R., and Vega, C., J. Chem. Phys., 2005, vol. 122, p. 234511.
Waage, M.H., Vlugt, T.J.H., and Kjelstrup, S., J. Phys. Chem. B, 2017, vol. 121, p. 7336.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 18-03-00654 A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of i-nterest.
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Sizova, A.A., Sizov, V.V. & Brodskaya, E.N. Molecular Dynamics Simulation of the Stability of Spherical Nanoclusters of Methane and Carbon Dioxide Hydrates. Colloid J 82, 180–187 (2020). https://doi.org/10.1134/S1061933X2002012X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X2002012X