Abstract
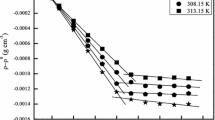
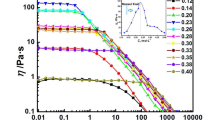
The structure and rheological behavior of a reactive oligomeric ionic liquid (OIL) have been studied. The OIL has a linear structure and contains ionic fragments of two types at both ends of oligo(ethylene oxide) chain. The ionic fragments are represented by secondary amino groups and nitrogen-containing heterocycles protonated with ethane sulfonic acid. The results obtained using rotational rheometry in different dynamic regimes indicate that, in the linear region of deformation at temperatures T < 20°C, this OIL exhibits the behavior of an elastic solidlike body. The components of the complex shear modulus (G ' ~ 107 Pa and G '' ~ 106 Pa) are independent of frequency ω and temperature. At the same time, its complex dynamic viscosity is independent of temperature and decreases with an increase in ω (in logarithmic coordinates, the dependence is linear and has a slope close to unity, which is a formal sign of the existence of a yield stress). At T ≤ 20°C in the nonlinear region of periodic deformation, a crossover is observed in the amplitude dependences of G ' and G '', which indicates that the critical shear stress is reached. As a result, the OIL passes into the liquid state (G '' > G '). The boundary, which is located at nearly 30°C, is characterized by equality between G ' and G '' in a wide range of strain amplitudes. The structural transformations induced by thermal and mechanical energies have been explained using a hypothesis of the existence of micellar structures and a “normal micelle–reverse micelle” transition in the OIL, as well as changes in micelle shapes. The analysis of the temperature dependences for the viscoelastic characteristics and scattered light intensity, as well as the data of DSC and optical microscopy, has led to the hypothesis that at, T = 21–28°C, the OIL may occur in an ordered state similar to the liquid-crystalline one.








Similar content being viewed by others
REFERENCES
Ionic Liquids: Theory, Properties, New Approaches, Kokorin, A., Ed., Croatia: In Tech, 2011.
Tarasova, N.P., Smetannikov, Yu.V., and Zanin, A.A., Usp. Khim., 2010, vol. 79, p. 516.
Zherenkova, L.V. and Komarov, P.V., Polym. Sci., Ser. A, 2014, vol. 56, p. 383.
Smirnova, N.A., Usp. Khim., 2005, vol. 74, p. 138.
Rusanov, A.I., Mitselloobrazovanie v rastvorakh po-verkhnostno-aktivnykh veshchestv (Micellization in Surfactant Solutions), St. Petersburg: Khimiya, 1992.
Rusanov, A.I., Colloid J., 2014, vol. 76, p. 121.
Rusanov, A.I., Shchekin, A.K., and Kuni, F.M., Colloid J., 2009, vol. 71, p. 816.
Rusanov A.I., Shchekin A.K., and Kuni F.M., Colloid J., 2009, vol. 71, p. 826.
Kuznetsov, V.S., Blinov, A.P., Usol’tseva, N.V., and Anan’eva, G.A., Colloid J., 2007, vol. 69, p. 627.
Kuznetsov, V.S., Usol’tseva, N.V., Zherdev, V.P., and Bykova, V.V., Colloid J., 2009, vol. 71, p. 784.
Kuznetsov, V.S., Usol’tseva, N.V., Zherdev, V.P., and Bykova, V.V., Colloid J., 2010, vol. 72, p. 216.
Safonova, E.A., Alexeeva, M.V., and Smirnova, N.A., Colloid J., 2009, vol. 71, p. 717.
Maeda, H., J. Phys. Chem. B, 1997, vol. 101, p. 7378.
Ikeda, S., Tsunoda, M., and Maeda, H., J. Colloid Interface Sci., 1979, vol. 70, p. 448.
Kaimoto, H., Shoho, K., Sasaki, S., and Maeda, H., J. Phys. Chem., 1994, vol. 98, p. 10243.
Goossens, K., Lava, K., Bielawski, C.W., and Binnemans, K., Chem. Rev., 2016, vol. 116, p. 4643.
Binnemans, K., Chem. Rev., 2005, vol. 105, p. 4148.
Godovskii, Yu.K. and Papkov, V.S., in Zhidkokristallicheskie polimery (Liquid Crystal Polymers), Plate, N.A., Ed., Moscow: Khimiya, 1988.
Pebalk, D.A., Barmatov, E.B., and Shibaev, V.P., Usp. Khim., 2005, vol. 74, p. 610.
Mezhikovskii, S.M., Arinshtein, A.E., and Deber-deev, R.Yu., Oligomernoe sostoyanie veshchestva (Oligomer State of Matter), Moscow: Nauka, 2005.
Shevchenko, V.V., Stryutsky, A.V., Klymenko, N.S., Gumenna, M.A., Fomenko, A.A., Bliznyuk, V.N., Trachevsky, V.V., Davydenko, V.V., and Tsukruk, V.V., Polymer, 2014, vol. 55, p. 3349.
Shevchenko, V.V., Stryutsky, A.V., Klymenko, N.S., Gumennaya, M.A., Fomenko, A.A., Trachevsky, V.V., Davydenko, V.V., Bliznyuk, V.N., and Dorokhin, A.V., Polym. Sci., Ser. B, 2014, vol. 56, p. 583.
Xu, W., Ledin, P.A., Shevchenko, V.V., and Tsukruk, V.V., ACS Appl. Mater. Interfaces, 2015, vol. 7, p. 12570.
Shevchenko, V.V., Gumennaya, M.A., Stryutskii, A.V., Klimenko, N.S., Trachevskii, V.V., Klepko, V.V., and Davydenko, V.V., Polym. Sci., Ser. B, 2018, vol. 60, p. 598.
Greaves, T.L. and Drummond, C.J., Chem. Rev., 2015, vol. 115, p. 11379.
Hayes, R., Warr, G.G., and Atkin, R., Chem. Rev., 2015, vol. 115, p. 6357.
Yuan, J., Meccerreyes, D., and Antonietti, M., Prog. Polym. Sci., 2013, vol. 38, p. 1009.
Shaplov, A.S., Ponkratov, D.O., Vlasov, P.S., Lozinskaya, E.I., Komarova, L.I., Malyshkina, I.A., Vidal, F., Nguyen, G.T.M., Armand, M., Wandrey, C., and Vygodskii, Ya.S., Polym. Sci., Ser. B, 2013, vol. 55, p. 122.
Shaplov, A.S., Ponkratov, D.O., and Vygodskii, Ya.S., Polym. Sci., Ser. B, 2016, vol. 58, p. 73.
Malkin, A.Ya., Semakov, A.V., and Kulichikhin, V.G., Polym. Sci., Ser. A, 2010, vol. 52, p. 1083.
Zapol’skii, A.K. and Baran, A.A., Koagulyanty i flokulyanty v protsessakh ochistki vody (Coagulants and Flocculants in Water Treatment Processes), Leningrad: Khimiya, 1987.
Geller, B.E., Geller, A.A., and Chertulov, V.G., Prakticheskoe rukovodstvo po fizikokhimii voloknoobrazuyushchikh polimerov (A Practical Guide on Physical Chemistry of Fiber-Forming Polymers), Moscow: Khimiya, 1996.
Shevchenko, V.V., Gumenna, M.A., Korolovych, V.F., Stryutsky, A.V., Trachevsky, V.V., Hrebnov, O., Klepko, V.V., Klymenko, N.S., Shumsky, V.F., Davyden-ko, V.V., and Ledin, P.A., J. Mol. Liq., 2017, vol. 235, p. 68.
Masalova, I., Taylor, M., Kharatiyan, E., and Malkin, A.Ya., J. Rheol. (NY), 2005, vol. 49, p. 839.
Malkin, A.Ya. and Isaev, A.I., Reologiya: kontseptsii, metody, prilozheniya (Rheology: Concepts, Methods, Applications), St. Petersburg: Professiya, 2010.
Reich, S. and Cohen, Y., J. Polym. Sci., Part B: Polym. Phys., 1981, vol. 19, p. 1255.
Il’in, S.O., Spiridonova, V.M., Savel’eva, V.S., Ovchinnikov, M.M., Khizhnyak, S.D., Frenkin, E.I., Pakhomov, P.M., and Malkin, A.Ya., Colloid J., 2011, vol. 73, p. 688.
Ilyin, S., Roumyantseva, T., Spiridonova, V., Semakov, A., Frenkin, E., Malkin, A., and Kulichikhin, V., Soft Matter, 2011, vol. 7, p. 9090.
Sun, L., Morales-Collazo, O., Xia, H., and Brennecke, J.F., J. Phys. Chem. B, 2015, vol. 119, p. 15030.
Sun, L., Morales-Collazo, O., Xia, H., and Brennecke, J.F., J. Phys. Chem. B, 2016, vol. 120, p. 5767.
Pulst, M., Samiullah, M.H., Baumeister, U., Prehm, M., Balko, J., Thurn-Albrecht, T., Busse, K., Golitsyn, Y., Reichert, D., and Kressler, J., Macromolecules, 2016, vol. 49, p. 6609.
Masalova, I., Malkin, A.Ya., and Foudazi, R., Appl. Rheol., 2008, vol. 18, p. 44790.
Nguyen, Q.D. and Boger, D.V., Rheol. Acta, 1985, vol. 24, p. 427.
Mewis, J., J. Non-Newtonian Fluid Mech., 1979, vol. 6, p. 1.
Papkov, S.P. and Kulichikhin, V.G., Zhidkokristallicheskoe sostoyanie polimerov (Liquid Crystalline State of Polymers), Moscow: Khimiya, 1977.
Kulichikhin, V.G., in Zhidkokristallicheskie polimery (Liquid Crystal Polymers), Plate, N.A., Ed., Moscow: Khimiya, 1988, p. 331.
Raghavan, S.R. and Khan, S.A., J. Rheol. (NY), 1995, vol. 39, p. 1311.
ACKNOWLEDGMENTS
We are grateful to V.G. Kulichikhin, corresponding member of the Russian Academy of Sciences, for valuable remarks and useful discussion of the obtained results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Shumskii, V.F., Shevchenko, V.V., Gumennaya, M.A. et al. Specific Features of the Rheological Behavior of a Protic Oligomeric Ionic Liquid of Cationic Type with Basic Sites of Two Types in the Region of the Solid–Liquid Transition. Colloid J 81, 804–816 (2019). https://doi.org/10.1134/S1061933X19050132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X19050132