Abstract
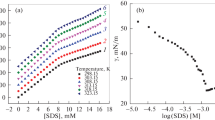
Proton chemical shifts of different atomic groups in sodium dodecyl sulfate (SDS) have been studied by 1H NMR spectroscopy as functions of surfactant concentration in aqueous solutions. Three surfactant concentration ranges of the chemical shifts have been revealed. The first range corresponds to the premicellar solutions, the second one is in the vicinity of critical micelle concentration (CMC1), and the third range corresponds to high surfactant concentrations, at which intermicellar interactions play a significant role. The parameters of SDS association (CMC1 and CMC2) determined based on the concentration dependences of the chemical shifts are in satisfactory agreement with the data available from the literature. The concept of critical dimerization concentration (CDC) has been introduced for the first concentration range. The values of CDC and dimerization constant K 2 (210 × 60 dm3/mol) have been estimated within the framework of the two-state model.
Similar content being viewed by others
References
Söderman, O., Stilbs, P., and Price, W.S., Concepts Magn. Reson., 2004, vol. 23, p. 121.
Pettersson, E., Topgaard, D., Stilbs, P., and Soderman, O., Langmuir, 2004, vol. 20, p. 1138.
Zuev, Yu.F., Gnezdilov, O.I., Zueva, O.S., and Us’yarov, O.G., Colloid J., 2011, vol. 73, p. 59.
Barhoum, S. and Yethiraj, A., J. Chem. Phys., 2010, vol. 132, p. 024909.
Pople, H.A., Schneider, W.G., and Bernstein, H.J., High-Resolution Nuclear Magnetic Resonance, New York: McGraw-Hill, 1959.
Stilbs, P., Prog. Nucl. Magn. Reson. Spectrosc., 1987, vol. 19, p. 1.
Soderman, O. and Stilbs, P., Prog. Nucl. Magn. Reson. Spectrosc., 1994, vol. 26, p. 445.
Qin, X., Liu, M., Yang, D., and Zhang, X., J. Phys. Chem. B, 2010, vol. 114, p. 3863.
Esposito, C., Colicchio, P., Facchiano, A., and Ragone, R., J. Colloid Interface Sci., 1998, vol. 200, p. 310.
Gu, G., Yan, H., Chen, W., and Wang, W., J. Colloid Interface Sci., 1996, vol. 178, p. 614.
Johnson, I., Olofsson, G., and Jonsson, B.J., J. Chem. Soc., Faraday Trans., 1987, vol. 83, p. 3331.
Joshi, T., Mata, J., and Bahadur, P., Colloids Surf. A, 2005, vol. 260, p. 209.
Cifuentes, A., Bernal, J., and Masa, D.J.C., Anal. Chem., 1997, vol. 69, p. 4271.
Bastiat, G., Grassl, B., Khoukh, A., and Francois, J., Langmuir, 2004, vol. 20, p. 759.
Zuev, Yu.F., Kurbanov, R.Kh., Idiyatullin, B.Z., and Us’yarov, O.G., Colloid J., 2007, vol. 69, p. 444.
Panicheva, L.P., Markina, Z.N., and Zadymova, N.M., Zh. Ross. Khim. O-va, 1989, no. 2, p. 245.
Panicheva, L.P., Cand. Sci. (Chem.) Dissertation, Moscow: Moscow State Univ., 1980.
Loginova, L.P., Visn. Kharkivsk. Nats. Univ., 2004, no. 626.
Podchasskaya, E.S. and Us’yarov, O.G., Colloid J., 2005, vol. 67, p. 177.
Rusanov, A.I., Mitselloobrazovanie v rastvorakh poverkhnostno-aktivnykh veshchestv (Micellization in Surfactant Solutions), St. Petersburg: Khimiya, 1992.
Gnezdilov, O.I., Zuev, Yu.F., Zueva, O.S., Patarikina, K.S., and Usyarov, O.G., Appl. Magn. Reson., 2011, vol. 40, p. 91.
Shah, S.S., Jamroz, N.U., and Sharif, Q.M., Colloids Surf. A, 2001, vol. 178, p. 199.
Dunstan, D.E. and White, L.R., J. Colloid Interface Sci., 1990, vol. 134, p. 147.
Mukerjee, P., Mysels, K.J., and Kapauan, D., J. Phys. Chem., 1967, vol. 71, p. 4166.
Podchasskaya, E.S. and Us’yarov, O.G., Colloid J., 2005, vol. 67, p. 184.
Burchfield, T.E. and Wooley, E.M., J. Phys. Chem., 1984, vol. 88, p. 3114.
Ekwall, P. and Mandell, L., J. Colloid Interface Sci., 1971, vol. 35, p. 519.
Movchan, T.G., Plotnikova, E.V., and Us’yarov, O.G., Colloid J., 2013, vol. 75, p. 319.
Quirion, F. and Magid, L.J., J. Phys. Chem., 1986, vol. 90, p. 5435.
Derjaguin, B.V., Churaev, N.V., and Muller, V.M., Surface Forces, New York: Consultants Bureau, 1987.
Us’yarov, O.G., Colloid J., 2007, vol. 69, p. 95.
Author information
Authors and Affiliations
Additional information
Original Russian Text © B.Z. Idiyatullin, K.S. Potarikina, Yu.F. Zuev, O.S. Zueva, O.G. Us’yarov, 2013, published in Kolloidnyi Zhurnal, 2013, Vol. 75, No. 5, pp. 585–590.
Rights and permissions
About this article
Cite this article
Idiyatullin, B.Z., Potarikina, K.S., Zuev, Y.F. et al. Association of sodium dodecyl sulfate in aqueous solutions according to chemical shifts in 1H NMR spectra. Colloid J 75, 532–537 (2013). https://doi.org/10.1134/S1061933X13050037
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X13050037