Abstract
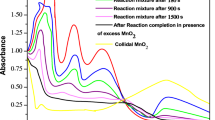
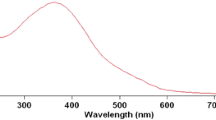
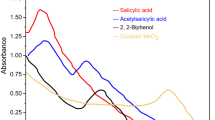
Kinetics of the title reaction has been studied spectrophotometrically in presence of perchloric acid at 30°C both in the absence and presence of Triton X-100 (TX-100). The reaction-time curves suggest the involvement of non-autocatalytic and autocatalytic reaction paths. The reaction follows first-order kinetics with respect to colloidal MnO2 and mandelic acid. The reaction has acid-dependent and acid-independent paths and, in the former case, the order is fractional in [H+]. Addition of nonionic surfactant (TX-100) catalysed the reaction which is explained on the basis of hydrogen bonding between the oxygen of polyoxyethylene chains of TX-100 and hydroxy groups of mandelic acid/colloidal MnO2. The kinetic data are rationalized in terms of model proposed by Tuncay et al. On the basis of the observed results, a possible mechanism has been proposed and discussed.
Similar content being viewed by others
References
Thomas, J.K., Chem. Rev., 1980, vol. 80, p. 283.
Sutin, N., in Eichorn, G.I. (Ed.), Inorganic Biochemistry, vol. 2, Amsterdam: Elsevier, 1973.
Piszkiewicz, D., J. Am. Chem. Soc., 1977, vol. 99, p. 7695.
Al-Lohedan, H.A., Al-Sulaim, A.M., Al-Ayed, A.S., and Issa, Z.A., J. Chem. Res.(S), 1993, p. 470.
Fendler, J.H., Membrane Mimetic Chemistry, New York: Wiley, 1982.
Rodriguez, A., Moya, M.L., Lopez, P., and Munoz, E., Int. J. Chem. Kinet., 2001, vol. 22, p. 1017; Rodriguez, A. and Moya, M.L., Langmuir, 1996, vol. 12, p. 4090.
Kabir-ud-Din, Hartani, K., and Khan, Z., Int. J. Chem. Kinet., 2001, vol. 33, p. 377; Colloids Surf., A, 2001, vol. 193, p. 1.
Sharma, T.C., Lal, A., and Saksena, V., Bull. Chem. Soc. Jpn., 1976, vol. 49, p. 288.
Kienzle, F., Tetrahedron Lett., 1983, vol. 24, p. 2213.
Taniguchi, S., Bull. Chem. Soc. Jpn., 1984, vol. 57, p. 2683.
Coughlin, R.W. and Matsui, I., J. Catal., 1976, vol. 41, p. 108.
Basak, B. and Malati, M.A., J. Inorg. Nucl. Chem., 1977, vol. 39, p. 1081.
Freeman, F. and Kappos, J.C., J. Am. Chem. Soc., 1985, vol. 107, p. 6628.
Perez-Benito, J.F. and Lee, D.G., Can. J. Chem., 1985, vol. 63, p. 1275.
Perez-Benito, J.F. and Arias, C., Int. J. Chem. Kinet., 1991, vol. 23, p. 717.
Perez-Benito, J.F., Brillas, E., and Pouplana, R., Inorg. Chem., 1989, vol. 28, p. 390.
Perez-Benito, J.F., Arias, C., and Amat, E., J. Colloid Interface Sci., 1996, vol. 177, p. 288.
Perez-Benito, J.F. and Arias, C., J. Colloid Interface Sci., 1992, vol. 149, p. 92.
Tuncay, M., Yuce, N., Arlkan, B., Gokturk, S., Colloids Surf., A, 1999, vol. 149, p. 279.
Khan, Z., Raju, Akram, M., and Kabir-ud-Din, Int. J. Chem. Kinet., 2004, vol. 36, p. 359.
Kabir-ud-Din, Fatma, W. and Khan, Z., Colloids Surf., A, 2004, vol. 234, p. 159.
Kabir-ud-Din, Iqubal, S.M.S., and Khan, Z., Colloid Polym. Sci., 2005, vol. 283, p. 504.
Kabir-ud-Din, Iqubal, S.M.S., and Khan, Z., Inorg. React. Mech., 2005, vol. 5, p. 151.
Kabir-ud-Din, Iqubal, S.M.S., and Khan, Z., Colloid Polym. Sci., 2005, vol. 284, p. 276.
Berne, B.J. and Pecora, R., Dynamic Light Scattering, New York: Wiley, 1976
Siddiqui, S.S., Ghosh, G., and Kabir-ud-Din, Langmuir, 2006, vol. 22, p. 9874.
Hasan, F. and Rocek, J., J. Am. Chem. Soc., 1972, vol. 94, p. 9073.
Pare, B., Pipada, B.A., Choube, A., and Bhagwat, V.W., Oxid. Commun., 2003, vol. 26, p. 95.
Wiberg, K.B. and Stewart, R., J. Am. Chem. Soc., 1955, vol. 77, p. 1786.
Senent, S. and Cuadrado, S., An. Quim., 1961, vol. 57, p. 11.
Macartney, D.H. and Sutin, N., Inorg. Chem., 1985, vol. 24, p. 3403.
Kabir-ud-Din, Morshed, A.M.A., and Khan, Z., Inorg. React. Mech., 2005, vol. 5, p. 151.
Kabir-ud-Din, Morshed, A.M.A., and Khan, Z., Carbohydr. Res., 2002, vol. 337, p. 1573.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Kabir-ud-Din, Shakeel Iqubal, S.M. Kinetics of the reduction of water soluble colloidal MnO2 by mandelic acid in the absence and presence of non-ionic surfactant triton X-100. Colloid J 72, 195–204 (2010). https://doi.org/10.1134/S1061933X10020080
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X10020080