Abstract
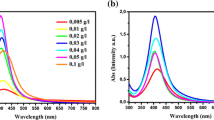
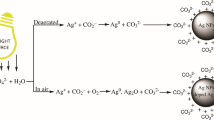
Comparative study of the properties of silver hydrosols prepared with the use of two classical procedures (“citrate” and “citrate-sulfate”) is performed. The possibility of using these procedures for the synthesis of stable monodisperse silver hydrosols with particle diameters of 20 nm and more is studied. The effect of the main parameters of synthesis (the ratio of initial components, the rate of their mixing, etc) on the hydrosol characteristics is investigated. It is revealed that, in the case of “citrate” synthesis, it is quite impossible to realize conditions ensuring the reproducible preparation of colloidal solutions with particles having sufficiently uniform size and shape. The procedure for the one-stage preparation of “citrate-sulfate” hydrosol (without multiple precipitation-redispersion of nanoparticles) is elaborated and it is shown that the thus prepared hydrosol is greatly superior in both the optical characteristics and the morphological uniformity of particles to the citrate sol. An increase in synthesis temperature to 100°C leads to a substantial enhancement of the stability of colloidal solution. The possibility of using “citrate-sulfate” hydrosol for the design of two-dimensional ensembles of silver nanoparticles on the quartz and silicon surfaces modified with poly(2-vinylpyridine) is demonstrated. It is shown that such ensembles possess optical properties that allow to use them in designing “two-dimensional” polymer-metal nanocomposites potentially suitable for using as active media in devices working on the principle of surface plasmon resonance.
Similar content being viewed by others
References
Lyon, L.A., Peňa, D.J., and Natan, M.J., J. Phys. Chem., 1999, vol. 103, p. 5826.
Maxwell, D.J., Emory, S.R., and Nie, S., Chem. Mater., 2001, vol. 13, p. 1082.
Roldughin, V.I., Usp. Khim., 2004, vol. 73, p. 123.
Protsenko, I.E., Starodubtsev, N.F., Rudoy, V.M., et al., Izv. Akad. Nauk, Ser. Fiz., 2006, vol. 70, p. 510.
Zhu, T., Fu, X., Mu, T., et al., Langmuir, 1999, vol. 15, p. 5197.
Auer, F., Scotti, M., Ulman, A., et al., Langmuir, 2000, vol. 16, p. 7554.
He, H.X., Zhang, H., Li, Q.G., et al., Langmuir, 2000, vol. 16, p. 3846.
Grabar, K.C., Freeman, R.G., Hommer, M.B., and Natan, M.J., Anal. Chem., 1995, vol. 67, p. 735.
Sato, T., Hasko, D.G., and Ahmed, H., J. Vac. Sci. Technol., B, 1997, vol. 15, p. 45.
Doron, A., Joselevich, E., Schlittner, A., and Willner, I., Thin Solid Films, 1999, vol. 340, p. 183.
Kooij, E.S., Wormeester, H., Brouwer, E.A.M., et al., Langmuir, 2002, vol. 18, p. 4401.
Bhat, R.R., Fisher, D.A., and Genzer, J., Langmuir, 2002, vol. 18, p. 5640.
Malynych, S., Lusinov, I., and Chumanov, G., J. Phys. Chem., B, 2002, vol. 106, p. 1280.
Schmitt, J., Mächtle, P., Eck, D., et al., Langmuir, 1999, vol. 15, p. 3256.
Dement’eva, O.V., Kartseva, M.E., Bol’shakova, A.V., et al., Kolloidn. Zh., 2005, vol. 67, p. 149.
Hrapovic, S., Liu, Y., Enright, G., et al., Langmuir, 2003, vol. 19, p. 3958.
Jiang, C., Markutsya, S., and Tsukruk, V.V., Langmuir, 2004, vol. 20, p. 882.
Zhao, S.-Y., Lei, S.-B., Chen, S.-H., et al., Colloid Polym. Sci., 2000, vol. 278, p. 682.
Turkevich, J., Stevenson, P.C., and Hillier, J., Discuss. Faraday Soc., 1951, vol. 11, p. 557.
Frens, G., Nature (London), 1973, vol. 211, p. 20.
Brown, K.R. and Natan, M.J., Langmuir, 1998, vol. 14, p. 726.
Daniel, M.-C. and Astruc, D., Chem. Rev., 2004, vol. 104, p. 293.
Mulvaney, P., Giersig, M., and Henglein, A., J. Phys. Chem., 1993, vol. 97, p. 7061.
Henglein, A. and Giersig, M., J. Phys. Chem., 1999, vol. 103, p. 9533.
Kapoor, S., Langmuir, 1998, vol. 14, p. 1021.
Ershov, B.G. and Henglein, A., J. Phys. Chem., 1993, vol. 97, p. 3434.
Yeung, S.A., Hobson, R., Biggs, S., and Grieser, F., J. Chem. Soc., Chem. Commun., 1993, p. 378.
Bell, W.C. and Myrick, M.L., J. Colloid Interface Sci, 2001, vol. 242, p. 300.
Tsuji, T., Iryo, K., Nishimura, Y., and Tsuji, M., J. Photochem. Photobiol., A, 2001, vol. 145, p. 201.
Rodriguez-Sanchez, L., Blanco, M.C., and Lopez-Quintela, M.A., J. Phys. Chem., B, 2000, vol. 104, p. 9683.
Silvert, P., Herrera-Urbina, R., and Tekaia-Elhsissen, K., J. Mater. Chem., 1997, vol. 7, p. 293.
Kurihara, L.K., Chow, G.M., and Schoen, P.E., Nanostruct. Mater., 1995, vol. 5, p. 607.
Fievet, F., Lagier, J.P., Blin, B., et al., Solid State Ionics, 1989, vols. 32–33, p. 198.
Ducamp-Sanguesa, C., Herrera-Urbina, R., and Figlarz, M., J. Solid State Chem., 1992, vol. 100, p. 272.
Luo, C., Zhang, Y., Zeng, X., et al., J. Colloid Interface Sci., 2005, vol. 288, p. 444.
Lin, X.Z., Teng, X., and Yang, H., Langmuir, 2003, vol. 19, p. 10081.
Cushing, B.L., Kolesnichenko, V.L., and O’Connor, C.J., Chem. Rev., 2004, vol. 104, p. 3893.
Nersisyan, H.H., Lee, J.H., Son, H.T., et al., Mater. Res. Bull., 2003, vol. 38, p. 949.
Shirtcliffe, N., Nickel, U., and Schneider, S., J. Colloid Interface Sci., 1999, vol. 211, p. 122.
Lee, P.C. and Meisel, D., J. Phys. Chem., 1982, vol. 86, p. 3391.
Pillai, Z.S. and Kamat, P.V., J. Phys. Chem., B, 2004, vol. 108, p. 945.
Munro, C.H., Smith, W.E., Garner, M., et al., Langmuir, 1995, vol. 11, p. 3712.
Cañamares, M.V., Garcia-Ramos, J.V., Gómez-Varga, J.D., et al., Langmuir, 2005, vol. 21, p. 8546.
Heard, S.M., Grieser, F., Barraclough, C.G., and Sanders, J.V., J. Colloid Interface Sci., 1983, vol. 93, p. 545.
Karpov, S.V., Popov, A.K., Slabko, V.V., and Shevnina, G.B., Kolloidn. Zh., 1995, vol. 57, p. 199.
Leopold, N. and Lendl, B., J. Phys. Chem., B, 2003, vol. 107, p. 5723.
Velikov, K.P., Zegers, G.E., and Van Blaaderen, A., Langmuir, 2003, vol. 19, p. 1384.
Schneider, S., Halbig, P., Grau, H., and Nickel, U., Photochem. Photobiol., 1994, vol. 60, p. 605.
LaMer, V.K. and Dinegar, R.H., J. Am. Chem. Soc., 1950, vol. 72, p. 4847.
Carey, L.M., Am. J. Sci., 1889, vol. 37, p. 476.
Frens, G. and Overbeek, J.Th.G., Kolloid Z. Z. Polym., 1969, vol. 233, p. 922.
Jolivet, J.P., Gzara, M., Mazieres, J., and Lefebvre, J., J. Colloid Interface Sci., 1985, vol. 107, p. 429.
Kim, K.Y., Choi, Y.T., Seo, D.J., and Park, S.B., Mater. Chem. Phys., 2004, vol. 88, p. 377.
Bogatyrev, V.A., Dykman, L.A., Khlebtsov, B.N., et al., Kolloidn. Zh., 2005, vol. 67, p. 458.
Filonov, A.S. and Yaminskii, I.V., Rukovodstvo pol’zovatelya paketa programmnogo obespecheniya dlya upravleniya skaniruyushchim zondovym mikroskopom i obrabotki izobrazhenii “FemtoSkan 001”. Versiya 2.16 (Guide for User of Software Package for Control of Scanning Probe Microscope and Image Processing “FemtoScan 001”. Version 2.16), Moscow: Tsentr Perspekt. Tekhnol., 1999.
Ershov, B.G. and Abkhalimov, E.A., Kolloidn. Zh., 2006, vol. 68, p. 459.
Sukhov, N.L., Ershov, B.G., Mikhalko, V.K., and Gordeev, A.V., Izv. Akad. Nauk, Ser. Khim., 1997, no. 1, p. 201.
Rivas, L., Sanchez-Cortes, S., Garcia-Ramos, J.V., and Morcillo, G., Langmuir, 2001, vol. 17, p. 574.
Ershov, B.G., Ross. Khim. Zh., 2001, vol. 45, p. 20.
Matijevič, E., Chem. Mater., 1993, vol. 5, p. 412.
Ledwith, D.M., Whelan, A.M., and Kelly, J.M., J. Mater. Chem., 2007, vol. 17, p. 2459.
Chen, S. and Carroll, D.L., Nano Lett., 2002, vol. 2, p. 1003.
Sun, Y. and Xia, Y., Adv. Mater. (Weinheim, Fed. Repub. Ger.), 2003, vol. 15, p. 695.
Fang, J., Huang, Y., Li, X., and Dou, X., Chem. Res. Chin. Univ., 2004, vol. 20, p. 817.
Sukhov, V.M., Dement’eva, O.V., Kartseva, M.E., et al., Kolloidn. Zh., 2004, vol. 66, p. 539.
Dement’eva, O.V., Filippenko, M.A., Pisarev, S.A., et al., Abstracts of Papers, XVIII Mendeleevskii s”ezd po obshchei i prikladnoi khimii (XVIII Mendeleev Congress on General and Applied Chemistry), Moscow, 2007, vol. 2, p. 215.
Author information
Authors and Affiliations
Additional information
Original Russian Text © O.V. Dement’eva, A.V. Mal’kovskii, M.A. Filippenko, V.M. Rudoy, 2008, published in Kolloidnyi Zhurnal, 2008, Vol. 70, No. 5, pp. 607–619.
Rights and permissions
About this article
Cite this article
Dement’eva, O.V., Mal’kovskii, A.V., Filippenko, M.A. et al. Comparative study of the properties of silver hydrosols prepared by “citrate” and “citrate-sulfate” procedures. Colloid J 70, 561–573 (2008). https://doi.org/10.1134/S1061933X08050050
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X08050050