Abstract
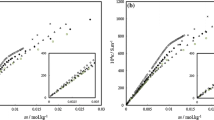
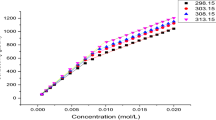
The concentration dependence of osmotic pressure πs of micellar solutions of an ionic surfactant in the presence of a background electrolyte is theoretically considered in terms of the Debye-Hückel theory with due regard for the premicellar association and interaction of micelles. On the basis of the quasi-chemical theory of micellization, the system composition is determined and the thickness of the electrical double layer of micelles is calculated. Within the framework of a cell model and the ideas of the molecular and ion-electrostatic interaction of micelles, which varies in relation to the degree of micellization, osmotic pressure in a sodium dodecyl sulfate-0.01 M NaCl system is calculated during variations in the overall surfactant concentrations. The results obtained are in good qualitative and quantitative agreement with available experimental data. At the same time, the results of calculating πs values in terms of the Debye-Hückel theory without consideration for the interaction of micelles do not allow explanation of the experimental regularities.
Similar content being viewed by others
References
Amos, D.A., Markels, J.H., Lynn, S., and Radke, C.J., J. Phys. Chem., B, 1998, vol. 102, p. 2739.
Lyonnard, S., Belloni, L., Reus, V., and Zemb, Th., J. Appl. Crystallogr., 2000, vol. 33, p. 582.
Reus, V., Belloni, L., Zemb, Th., et al., Colloids Surf., A, 1999, vol. 151, p. 449.
Activity Coefficients in Electrolyte Solutions, Pitzer, K.S., Ed., Boca Raton: CRC, 1991.
Alexander, S., Chaikin, P.M., Grant, P., et al., J. Chem. Phys., 1984, vol. 80, p. 5776.
Tieleman, D.P., Van der Spoel, D., and Berendsen, H.J.C., J. Phys. Chem., B, 2000, vol. 104, p. 6380.
Vlachy, V., Annu. Rev. Phys. Chem., 1999, vol. 50, p. 145.
Hribar, B. and Vlachy, V., J. Phys. Chem., B, 2000, vol. 104, p. 4218.
Rusanov, A.I., Mitselloobrazovanie v rastvorakh poverkhnostno-aktivnykh veshchestv (Micellization in Surfactant Solutions), St. Petersburg: Khimiya, 1992.
Rusanov, A.I., Kolloidn. Zh., 1998, vol. 60, p. 255.
Vervey, E.J. and Overbeek, I.Th.G., Theory of the Stability of Lyophobic Colloids, New York: Elsevier, 1948.
Derjaguin, B.V., Churaev, N.V., and Muller, V.M., Surface Forces, New York: Consultants Bureau, 1987.
Derjaguin, B.V., Teoriya ustoichivosti kolloidov i tonkikh plenok (The Theory of the Stability of Colloids and Thin Films), Moscow: Nauka, 1986.
Zharkikh, N.I. and Shilov, V.N., Kolloidn. Zh., 1982, vol. 44, p. 567.
Zharkikh, N.I. and Shilov, V.N., Kolloidn. Zh., 1982, vol. 44, p. 571.
Zholkovskij, E.K., Czarnecki, J., and Misliyah, J.H., J. Colloid Interface Sci., 2001, vol. 234, p. 293.
Zholkovskij, E.K., Dukhin, S.S., Mishchuk, N.A., et al., Colloids Surf., A, 2001, vol. 192, p. 235.
Bratko, P. and Vlachy, V., Colloid Polym. Sci., 1985, vol. 263, p. 417.
Alekseev, V.L., Semashko, O.V., and Us’yarov, O.G., Kolloidn. Zh., 2000, vol. 62, p. 293.
Corti, M. and Deglorgio, V., J. Phys. Chem., 1981, vol. 85, p. 711.
Zoeller, N. and Blankschtein, D., Langmuir, 1998, vol. 14, p. 7155.
Efremov, I.F. and Us’yarov, O.G., Usp. Khim., 1976, vol. 45, p. 877.
Schmitz, K.S., Langmuir, 2000, vol. 16, p. 2115.
Sanghiran, V. and Schmitz, K.S., Langmuir, 2000, vol. 16, p. 7566.
Panicheva, L.P., Cand. Sci. (Chem.) Dissertation, Moscow: Mosk. Gos. Univ., 1980.
Panicheva, L.P., Markina, Z.P., and Zadymova, N.M., Zh. Vses. Khim. O-va im. D.I. Mendeleeva, 1989, no. 2, p. 245.
Podchasskaya, E.S. and Us’yarov, O.G., Kolloidn. Zh., 2005, vol. 67, p. 206.
Carvey, C.J., Gilbert, E.P., Schulz, J.C., and Knott, R.B., Neutron News, 2003, vol. 14, p. 27.
Carsughi, F. and Schwahn, D., Physica B (Amsterdam), 1997, vols. 234–236, p. 343.
Us’yarov, O.G., Kolloidn. Zh., 2007, vol. 69, no. 1, p. 95.
Sonntag, H. and Strenge, K., Koagulation und Stabilitat Disperser Systeme, Berlin: Deutscher Verlag der Wissenschaften, 1970.
Israelachvili, J.N., Intermolecular and Surface Forces, London: Academic, 1991, 2nd ed., p. 190.
Van den Tempel, M., J. Colloid Sci., 1958, vol. 39, p. 125.
Dukhin, S.S., Derjaguin, B.V., and Semenikhin, N.M., Dokl. Akad. Nauk SSSR, 1970, vol. 192, p. 357.
Hoskin, N.E. and Levine, S., Philos. Trans. R. Soc. London, A, 1956, vol. 248, p. 345.
Author information
Authors and Affiliations
Additional information
Original Russian Text © O.G. Us’yarov, 2007, published in Kolloidnyi Zhurnal, 2007, Vol. 69, No. 1, pp. 118–123.
Rights and permissions
About this article
Cite this article
Us’yarov, O.G. Osmotic pressure of micellar sodium dodecyl sulfate solutions in the presence of NaCl. Colloid J 69, 111–116 (2007). https://doi.org/10.1134/S1061933X07010152
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X07010152