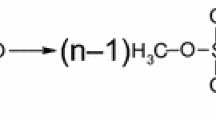Abstract
Polysiloxane xerogels containing 3-mercaptopropyl and methyl groups in the surface layer were synthesized by the sol-gel method with ethanol used as a solvent and fluoride ion used as a catalyst. It is established that an increase in the relative content of methyltriethoxysilane in the initial reaction mixture results in formation of xerogels with a developed porous structure. The tendency for an increase in other characteristics of porous structure, the sorption volume and pore size, is also observed. The analogous effect is found upon increasing relative content of tetraethoxysilane with a constant ratio between two trifunctional silanes. By means of atomic force microscopy, it is shown that the xerogels are composed of aggregated particles with mean sizes of 35–45 nm. These results correlate with the data of scanning electron microscopy. On the basis of the data of IR spectroscopy and 13C CP/MAS NMR spectroscopy, it is concluded that the surface layers contain not only 3-mercaptopropyl and methyl groups but also silanol groups, a part of the unhydrolyzed alkoxy groups, and water molecules involved in the formation of hydrogen bonds. The results obtained by 29Si CP/MAS NMR spectroscopy testify that, in synthesized xerogels, the structural units of T1 type [(≡SiO)Si(OR′)2CH3 and/or (≡SiO)Si(OR′)2(CH2)3SH, R′ = H, OCH3 or OC2H5] are absent and the structural units of T3 type [(≡SiO)3SiCH3 and (≡SiO)3Si(CH2)3SH] dominate compared to the units of T2 type [(≡SiO)2Si(OR′)CH3 and (≡SiO)2Si(OR′)(CH2)3SH]. These results are an indirect indication of enhanced hydrolytic stability of surface layers in such xerogels.
Similar content being viewed by others
References
Functional Hybrid Materials, Gomez-Romero, P. and Sanchez, C., Eds., Weinheim: Wiley-VCH, 2004.
Handbook of Sol-Gel Science and Technology: Processing, Characterization, and Applications, vols. 1–3, Sakka, S., Ed., Dordrecht: Kluwer, 2005.
Slinyakova, I.B. and Denisova, T.I., Kremniiorganicheskie adsorbenty. Poluchenie, sovistva, primenenie (Organosilicon Adsorbents: Synthesis, Properties, Applications), Kiev: Naukova Dumka, 1988.
Zub, Yu.L. and Parish, R.V., Stud. Surf. Sci. Catal., 1996, vol. 99, p. 285.
Avnir, D., Klein, L.C., Levy, D., et al., in The Chemistry of Organic Silicon Compounds, Rappoport, Z. and Apeloig, Y., Eds., New York: Wiley, 1998, vol. 2, p. 2317.
Voronkov, M.G., Vlasova, N.N., and Pozhidaev, Yu.N., Appl. Organomet. Chem., 2000, vol. 14, p. 287.
El-Nahhal, I.M., Yang, J.J., Chuang, I.-S., and Maciel, G.E., J. Non-Cryst. Solids, 1996, vol. 208, p. 105.
Yang, J.J., El-Nahhal, I.M., Chuang, I.-S., and Maciel, G.E., J. Non-Cryst. Solids, 1997, vol. 212, p. 281.
Zub, Yu.L., Chuiko, A.A., and Stechenko, E.V., Dokl. Akad. Nauk Ukr., 2002, no. 4, p. 150.
Melnyk (Seredyuk), I.V., Zub, Yu.L., Chuiko, A.A., and Van der Voort, P., Chem. Phys. Technol. Surf., 2002, vol. 8, p. 125.
Zub, Yu.L., Drozd, L.S., and Chuiko, A.A., Abstracts of Papers, IUPAC Symp. on Characterization of Porous Solids, Marseille, 1993, p. 95.
Zub, Yu.L. and Chuiko, A.A., in Colloidal Silica: Fundamentals and Applications, Bergna, H.E., Eds., Washington, DC: Marcel Dekker, 2005, p. 397.
Dobryanskaya, G.I., Mel’nik, I.V., Zub, Yu.L., et al., Abstracts of Papers, X Vserossiiskii simpozium s uchastiem inostrannykh uchenykh “Aktual’nye problemy teorii adsorbtsii, poristosti i adsorbtsionnoi selektivnosti” (X All-Russia Symp. with Participation of Foreign Scientists “Current Problems of Theory of Adsorption, Porosity, and Adsorption Selectivity”), Moscow-Klyaz’ma, 2005, p. 54.
Dobryanskaya, G.I., Mel’nik, I.V., Zub, Yu.L., et al., Zh. Fiz. Khim., 2006, vol. 80, no. 6.
Brunauer, J.S., Emmett, P.H., and Teller, E., J. Am. Chem. Soc., 1938, vol. 60, p. 309.
Barret, E.P., Joyner, L.G., and Halenda, P.P., J. Am. Chem. Soc., 1951, vol. 73, p. 373.
Gregg, S. and Sing, K.S.W., Adsorption, Surface Area and Porosity, New York: Academic, 1982.
Corriu, R.J.P., Perz, R., and Reye, C., Tetrahedron, 1983, vol. 39, p. 999.
Diaz, I., Mohino, F., Perez-Pariente, J., and Sastre, E., Thermochim. Acta, 2004, vol. 413, p. 201.
Finn, L.P. and Slinyakova, I.B., Kolloidn. Zh., 1975, vol. 37, p. 723.
Lin-Vien, D., Colthup, N.B., Fateley, W.G., and Grasselli, J.G., in The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules, London: Academic, 1991, p. 263.
Maciel, G.E., in Solid State NMR of Polymers, Ando, I. and Asakura, T., Eds., Amsterdam: Elsevier, 1998, p. 923.
Engelhardt, G. and Michel, D., High-Resolution Solid-State NMR of Silicates and Zeolites, Chichester: Wiley, 1987, p. 405.
Khatib, I.S. and Parish, R.V., J. Organomet. Chem., 1989, vol. 369, p. 9.
Sing, K.S.W., Everett, D.H., Haul, R.A.W., et al., Pure Appl. Chem., 1985, vol. 57, p. 603.
Author information
Authors and Affiliations
Additional information
Original Russian Text © G.I. Dobryanskaya, Yu.L. Zub, M. Barczak, A. Dabrowski, 2006, published in Kolloidnyi Zhurnal, 2006, Vol. 68, No. 5, pp. 601–611.
Rights and permissions
About this article
Cite this article
Dobryanskaya, G.I., Zub, Y.L., Barczak, M. et al. Synthesis and structure-related adsorption characteristics of bifunctional polysiloxane xerogels with methyl and 3-mercaptopropyl groups. Colloid J 68, 548–557 (2006). https://doi.org/10.1134/S1061933X06050048
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X06050048